2.1 INTRODUCTION
In this chapter we consider the structure of nerve and muscle tissue and in particular their membranes, which are excitable. A qualitative description of the activation process follows. Many new terms and concepts are mentioned only briefly in this chapter but in more detail in the next two chapters, where the same material is dealt with from a quantitative rather than a qualitative point of view.
These are described in Figure 2.1.
2. Transducer potentials across the membrane, due to external events. These include generator potentials caused by receptors or synaptic potential changes arising at synapses. Both subtypes can be inhibitory or excitatory.
3. As a consequence of transducer potentials, further response will arise. If the magnitude does not exceed the threshold, the response will be nonpropagating (electrotonic). If the response is great enough, a nerve impulse (action potential impulse) will be produced which obeys the all-or-nothing law (see below) and proceeds unattenuated along the axon or fiber.
After stimulation the membrane voltage returns to its original resting value.
A myelinated axon (surrounded by the myelin sheath) can produce a nerve impulse only at the nodes of Ranvier. In these axons the nerve impulse propagates from one node to another, as illustrated in Figure 2.12. Such a propagation is called saltatory conduction (saltare, "to dance" in Latin).
Berne RM, Levy MN (1993): Physiology, 3rd ed., 1091 pp. C. V. Mosby, St. Louis.
Bullock TH (1959): Neuron doctrine and electrophysiology. Science 129:(3355) 997-1002.
Davis LJ, Lorente de Nó R (1947): Contributions to the mathematical theory of the electrotonus. Stud. Rockefeller Inst. Med. Res. 131: 442-96.
Elsberg CA (1931): The Edwin Smith surgical papyrus. Ann. Med. Hist. 3: 271-9.
Ganong WF (1991): Review of Medical Physiology, 15th ed., Appleton & Lange, Norwalk, Conn.
Guyton AC (1992): Human Physiology and Mechanisms of Disease, 5th ed., 690 pp. Saunders, Philadelphia.
Hermann L (1872): Grundriss der Physiologie, 4th ed., (Quoted in L Hermann (1899): Zur Theorie der Erregungsleitung und der elektrischen Erregung. Pflüger Arch. ges. Physiol. 75: 574-90.)
Hermann L (1905): Lehrbuch der Physiologie, 13th ed., 762 pp. August Hirschwald, Berlin.
Kandel ER, Schwartz JH (1985): Principles of Neural Science, Elsevier Publishing, New York.
Lorente de Nó R (1947): A Study of Nerve Physiology, 293 pp. Rockefeller Institute for Medical Research, New York.
Muler AL, Markin VS (1978): Electrical properties of anisotropic nerve-muscle syncytia - II. Spread of flat front of excitation. Biophys. 22: 536-41.
Nunez PL (1981): Electric Fields of the Brain: The Neurophysics of EEG, 484 pp. Oxford University Press, New York.
Patton HD, Fuchs AF, Hille B, Scher AM, Steiner R (eds.) (1989): Textbook of Physiology, 21st ed., 1596 pp. W. B. Saunders, Philadelphia.
Ruch TC, Patton HD (eds.) (1982): Physiology and Biophysics, 20th ed., 1242 pp. W. B. Saunders, Philadelphia.
Schadé JP, Ford DH (1973): Basic Neurology, 2nd ed., 269 pp. Elsevier Scientific Publishing, Amsterdam.
Thompson CF (1985): The Brain - An Introduction to Neuroscience, 363 pp. W. H. Freeman, New York.
2.2 NERVE CELL
2.2.1 The Main Parts of the Nerve Cell
The nerve cell may be divided on the basis of its structure and function into three main parts:
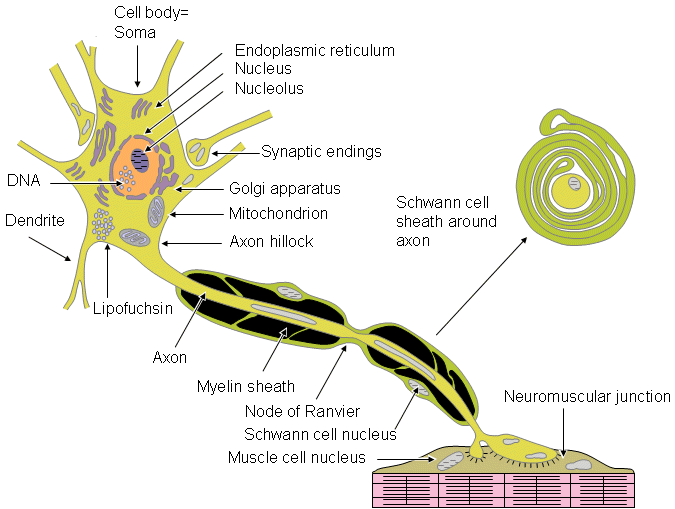
Fig. 2.1. The major components of a neuron.
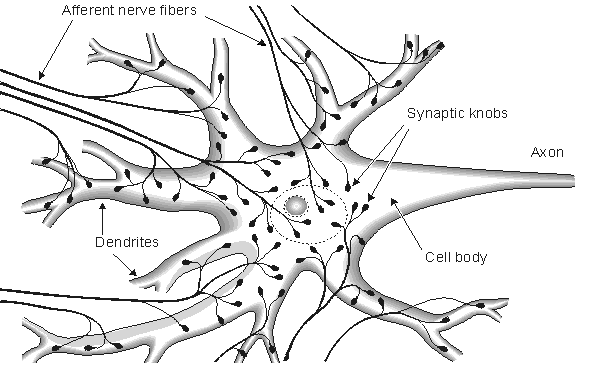
Fig. 2.2. Cortical nerve cell and nerve endings connected to it.
2.2.2 The Cell Membrane
The cell is enclosed by a cell membrane whose thickness is about 7.5 - 10.0 nm. Its structure and composition resemble a soap-bubble film (Thompson, 1985), since one of its major constituents, fatty acids, has that appearance. The fatty acids that constitute most of the cell membrane are called phosphoglycerides. A phosphoglyceride consists of phosphoric acid and fatty acids called glycerides (see Figure 2.3). The head of this molecule, the phosphoglyceride, is hydrophilic (attracted to water). The fatty acids have tails consisting of hydrocarbon chains which are hydrophobic (repelled by water).
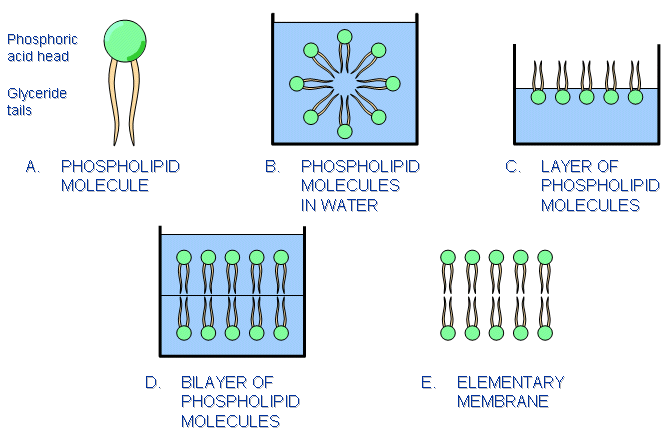
Fig. 2.3. A sketch illustrating how the phosphoglyceride (or phospholipid) molecules behave in water. See text for discussion.
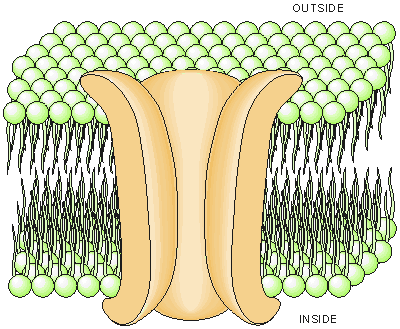
Fig. 2.4. The construction of a cell membrane. The main constituents are two lipid layers, with the hydrophobic tails pointing inside the membrane (away from the aqueous intracellular and interstitial mediums). The macromolecular pores in the cell membrane form the ionic channels through which sodium, potassium, and chloride molecules flow through the membrane and generate the bioelectric phenomena.
2.2.3 The Synapse
The junction between an axon and the next cell with which it communicates is called the synapse. Information proceeds from the cell body unidirectionally over the synapse, first along the axon and then across the synapse to the next nerve or muscle cell. The part of the synapse that is on the side of the axon is called the presynaptic terminal; that part on the side of the adjacent cell is called the postsynaptic terminal. Between these terminals, there exists a gap, the synaptic cleft, with a thickness of 10 - 50 nm. The fact that the impulse transfers across the synapse only in one direction, from the presynaptic terminal to the postsynaptic terminal, is due to the release of a chemical transmitter by the presynaptic cell. This transmitter, when released, activates the postsynaptic terminal, as shown in Figure 2.5. The synapse between a motor nerve and the muscle it innervates is called the neuromuscular junction. Information transfer in the synapse is discussed in more detail in Chapter 5.
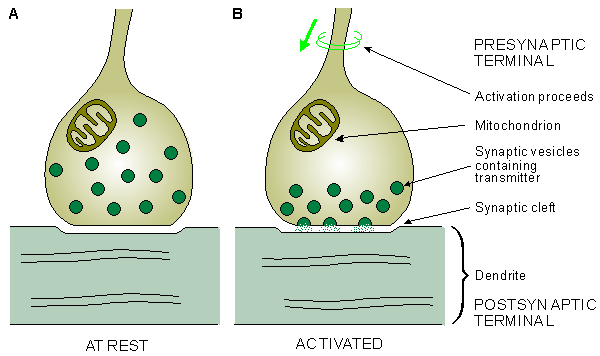
Fig. 2.5. Simplified illustration of the anatomy of the synapse.
(A) The synaptic vesicles contain a chemical transmitter.
(B) When the activation reaches the presynaptic terminal the transmitter is released and it diffuses across the synaptic cleft to activate the postsynaptic membrane.2.3 MUSCLE CELL
There are three types of muscles in the body:
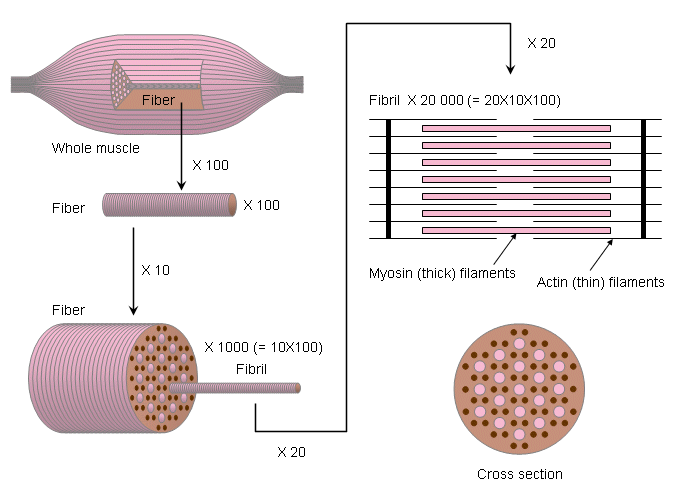
Fig. 2.6. Anatomy of striated muscle. The fundamental physiological unit is the fiber.
2.4 BIOELECTRIC FUNCTION OF THE NERVE CELL
The membrane voltage (transmembrane voltage) (Vm) of an excitable cell is defined as the potential at the inner surface (Fi) relative to that at the outer (Fo) surface of the membrane, i.e. Vm = (Fi) - (Fo). This definition is independent of the cause of the potential, and whether the membrane voltage is constant, periodic, or nonperiodic in behavior. Fluctuations in the membrane potential may be classified according to their character in many different ways. Figure 2.7 shows the classification for nerve cells developed by Theodore Holmes Bullock (1959). According to Bullock, these transmembrane potentials may be resolved into a resting potential and potential changes due to activity. The latter may be classified into three different types:
1. Pacemaker potentials: the intrinsic activity of the cell which occurs without external excitation.
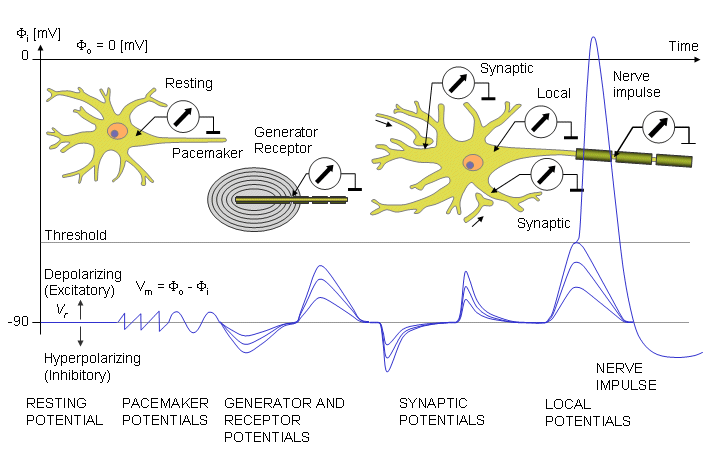
Fig. 2.7. Transmembrane potentials according to Theodore H. Bullock.
2.5 EXCITABILITY OF NERVE CELL
If a nerve cell is stimulated, the transmembrane voltage necessarily changes. The stimulation may be
excitatory (i.e., depolarizing; characterized by a change of the potential inside the cell relative to the outside in the positive direction, and hence by a decrease in the normally negative resting voltage) or
inhibitory (i.e., hyperpolarizing, characterized by a change in the potential inside the cell relative to the outside in the negative direction, and hence by an increase in the magnitude of the membrane voltage).
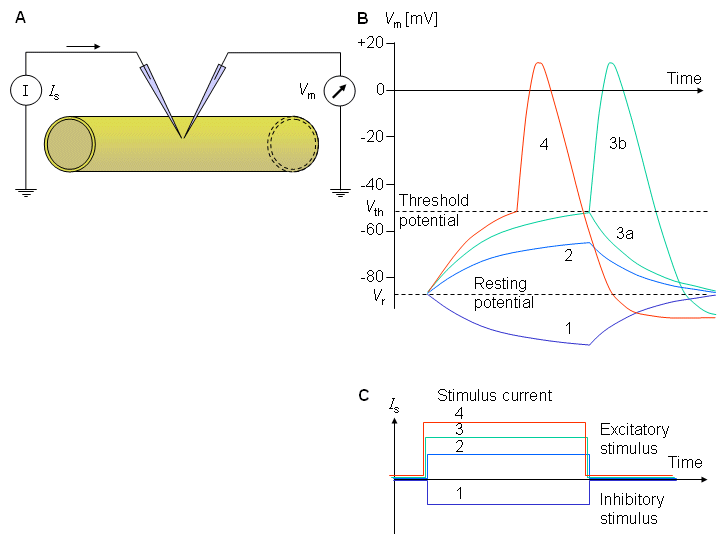
Fig. 2.8. (A) Experimental arrangement for measuring the response of the membrane potential (B) to inhibitory (1) and excitatory (2, 3, 4) stimuli (C). The current stimulus (2), while excitatory is, however, subthreshold, and only a passive response is seen. For the excitatory level (3), threshold is marginally reached; the membrane is sometimes activated (3b), whereas at other times only a local response (3a) is seen. For a stimulus (4), which is clearly transthreshold, a nerve impulse is invariably initiated.
2.6 THE GENERATION OF THE ACTIVATION
The mechanism of the activation is discussed in detail in Chapter 4 in connection with the Hodgkin-Huxley membrane model. Here the generation of the activation is discussed only in general terms.
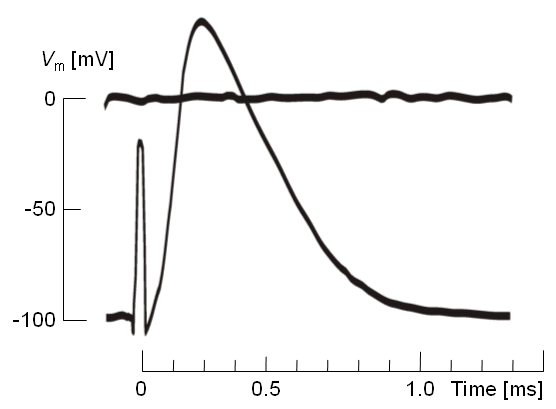
Fig. 2.9. Nerve impulse recorded from a cat motoneuron following a transthreshold stimulus. The stimulus artifact may be seen at t = 0.
2.7 CONCEPTS ASSOCIATED WITH THE ACTIVATION PROCESS
Some basic concepts associated with the activation process are briefly defined in this section. Whether an excitatory cell is activated depends largely on the strength and duration of the stimulus. The membrane potential may reach the threshold by a short, strong stimulus or a longer, weaker stimulus. The curve illustrating this dependence is called the strength-duration curve; a typical relationship between these variables is illustrated in Figure 2.10. The smallest current adequate to initiate activation is called the rheobasic current or rheobase. Theoretically, the rheobasic current needs an infinite duration to trigger activation. The time needed to excite the cell with twice rheobase current is called chronaxy.
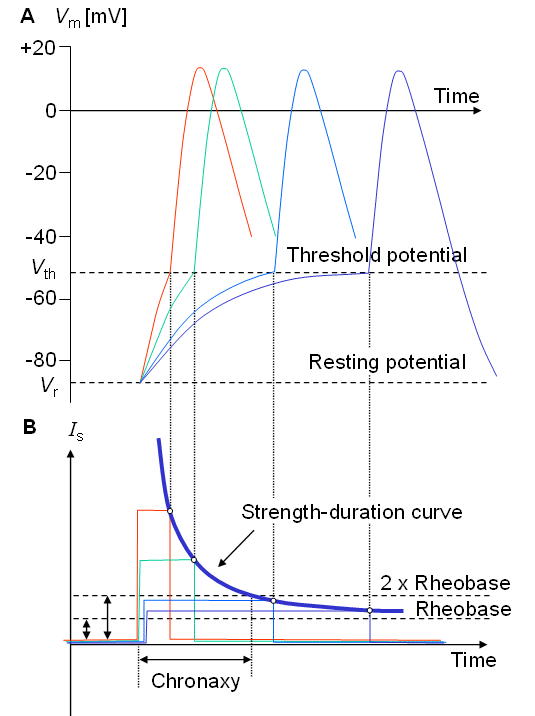
Fig. 2.10. (A) The response of the membrane to various stimuli of changing strength (B), the strength-duration curve. The level of current strength which will just elicit activation after a very long stimulus is called rheobase. The minimum time required for a stimulus pulse twice the rheobase in strength to trigger activation is called chronaxy. (For simplicity, here, threshold is shown to be independent on stimulus duration.)
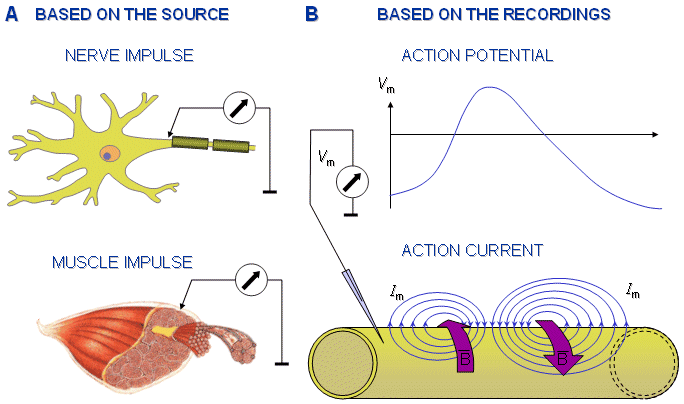
Fig. 2.11. Clarification of the terminology used in connection with the action impulse:
A) The source of the action impulse may be nerve or muscle cell. Correspondingly it is called a nerve impulse or a muscle impulse.
B) The electric quantity measured from the action impulse may be potential or current. Correspondingly the recording is called an action potential or an action current.
2.8 CONDUCTION OF THE NERVE IMPULSE IN AN AXON
Ludvig Hermann (1872, 1905) correctly proposed that the activation propagates in an axon as an unattenuated nerve impulse. He suggested that the potential difference between excited and unexcited regions of an axon would cause small currents, now called local circuit currents, to flow between them in such a direction that they stimulate the unexcited region.

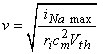
(2.1)
where v = velocity of the nerve impulse [m/s] iNa max = maximum sodium current per unit length [A/m] Vth = threshold voltage [V] ri = axial resistance per unit length [W/m] cm = membrane capacitance per unit length [F/m]
v = 6d (2.2)
where v = velocity [m/s] d = axon diameter [µm] 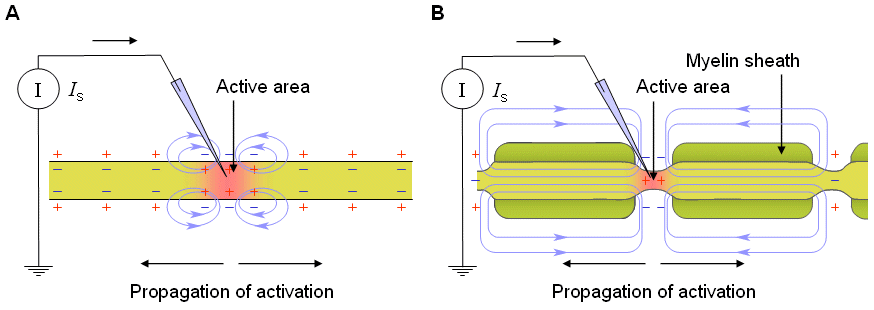
Fig. 2.12. Conduction of a nerve impulse in a nerve axon.
(A) continuous conduction in an unmyelinated axon;
(B) saltatory conduction in a myelinated axon.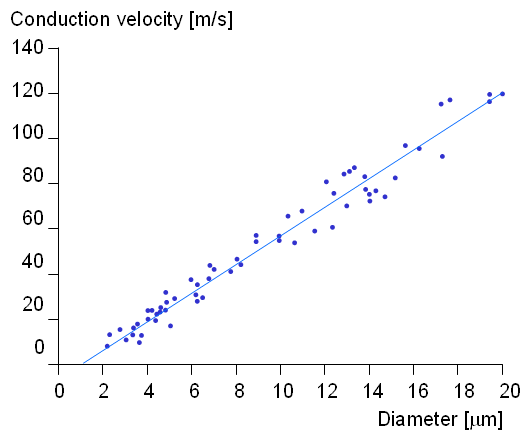
Fig. 2.13. Experimentally determined conduction velocity of a nerve impulse in a mammalian myelinated axon as a function of the diameter. (Adapted from Ruch and Patton, 1982.)
REFERENCES


