5.1 INTRODUCTION
The focus of this book is primarily the electric activity of nerve and muscle and the extracellular electric and magnetic fields that they generate. It is possible to undertake such a study without considering the functional role of nerve and muscle in physiology. But without some life science background, the reader's evaluation of electrophysiological signals would necessarily be handicapped. For that reason, we have included an overview, with appropriate terminology, of relevant topics in physiology. This chapter is therefore devoted to a survey of the organization of the nervous system and its main components. It is hoped that the reader will find it helpful for understanding of the physiological function of the excitable tissues discussed in other chapters, and to know what to look for elsewhere. For further study, we suggest the following texts: Jewett and Rayner (1984); Kuffler, Nicholls, and Martin (1984); Nunez (1981); Patton et al. (1989); Schmidt (1981); Shepherd (1988); all of which appear in the list of references.
 A discussion of the nervous system might logically begin with sensory cells located at the periphery of the body. These cells initiate and conduct signals to the brain and provide various sensory inputs such as vision, hearing, posture, and so on. Providing information on the environment to the body, these peripheral cells respond to stimuli with action pulses, which convey their information through encoded signals. These signals are conducted axonally through ascending pathways, across synapses, and finally to specific sites in the brain. Other neural cells in the brain process the coded signals, and direct the actions of muscles and other organs in response to the various sensory inputs. The entire circuit is recognized as a reflex arc, a basic unit in the nervous system. In some cases it is entirely automatic, and in others it is under voluntary control.
A discussion of the nervous system might logically begin with sensory cells located at the periphery of the body. These cells initiate and conduct signals to the brain and provide various sensory inputs such as vision, hearing, posture, and so on. Providing information on the environment to the body, these peripheral cells respond to stimuli with action pulses, which convey their information through encoded signals. These signals are conducted axonally through ascending pathways, across synapses, and finally to specific sites in the brain. Other neural cells in the brain process the coded signals, and direct the actions of muscles and other organs in response to the various sensory inputs. The entire circuit is recognized as a reflex arc, a basic unit in the nervous system. In some cases it is entirely automatic, and in others it is under voluntary control.
 No neurons run directly from the periphery to the brain. Normally the initiated signal is relayed by several intermediate neural cells. The interconnection between neurons, called the synapse, behaves as a simple switch but also has a special role in information processing. The junction (synapse) between a neural cell and the muscle that it innervates, called the neuromuscular junction, has been particularly well studied and provides much of our quantitative understanding about synapses. Since it is impossible to discuss the structure of the nervous system without including synapses, we begin our discussion with an examination of that topic.
No neurons run directly from the periphery to the brain. Normally the initiated signal is relayed by several intermediate neural cells. The interconnection between neurons, called the synapse, behaves as a simple switch but also has a special role in information processing. The junction (synapse) between a neural cell and the muscle that it innervates, called the neuromuscular junction, has been particularly well studied and provides much of our quantitative understanding about synapses. Since it is impossible to discuss the structure of the nervous system without including synapses, we begin our discussion with an examination of that topic.
5.2 SYNAPSES
5.2.1 Structure and Function of the Synapse
The function of the synapse is to transfer electric activity (information) from one cell to another. The transfer can be from nerve to nerve (neuro-neuro), or nerve to muscle (neuro-myo). The region between the pre- and postsynaptic membrane is very narrow, only 30-50 nm. It is called the synaptic cleft (or synaptic gap). Direct electric communication between pre- and postjunctional cells does not take place; instead, a chemical mediator is utilized. The sequence of events is as follows:
- An action pulse reaches the terminal endings of the presynaptic cell.
- A neurotransmitter is released, which diffuses across the synaptic gap to bind to receptors in specialized membranes of the postsynaptic cell.
- The transmitter acts to open channels of one or several ion species, resulting in a change in the transmembrane potential. If depolarizing, it is an excitatory postsynaptic potential (EPSP); if hyperpolarizing, an inhibitory postsynaptic potential (IPSP).
Figure 5.1 shows the synapse between a nerve and muscle cell, a neuromuscular junction.
 In cardiac muscle the intercellular space between abutting cells is spanned by gap junctions, which provide a low-resistance path for the local circuit currents and may be regarded as an electric (myo-myo) synapse. (The gap, however, is not called a synaptic cleft.) This type of junction is discussed in a later chapter.
In cardiac muscle the intercellular space between abutting cells is spanned by gap junctions, which provide a low-resistance path for the local circuit currents and may be regarded as an electric (myo-myo) synapse. (The gap, however, is not called a synaptic cleft.) This type of junction is discussed in a later chapter.
 The presynaptic nerve fiber endings are generally enlarged to form terminal buttons or synaptic knobs. Inside these knobs are the vesicles that contain the chemical transmitters. The arrival of the action pulse opens voltage-gated Ca2+ channels that permit an influx of calcium ions. These in turn trigger the release into the synaptic gap, by exocytosis, of a number of the "prepackaged" vesicles containing the neurotransmitter.
The presynaptic nerve fiber endings are generally enlarged to form terminal buttons or synaptic knobs. Inside these knobs are the vesicles that contain the chemical transmitters. The arrival of the action pulse opens voltage-gated Ca2+ channels that permit an influx of calcium ions. These in turn trigger the release into the synaptic gap, by exocytosis, of a number of the "prepackaged" vesicles containing the neurotransmitter.
 On average, each neuron divides into perhaps 1000 synaptic endings. On the other hand, a single spinal motor neuron may have an average of 10,000 synaptic inputs. Based on this data, it is not surprising that the ratio of synapse to neurons in the human forebrain is estimated to be around 4×104. In neuro-neuro synapses, the postjunctional site may be a dendrite or cell body, but the former predominates.
On average, each neuron divides into perhaps 1000 synaptic endings. On the other hand, a single spinal motor neuron may have an average of 10,000 synaptic inputs. Based on this data, it is not surprising that the ratio of synapse to neurons in the human forebrain is estimated to be around 4×104. In neuro-neuro synapses, the postjunctional site may be a dendrite or cell body, but the former predominates.
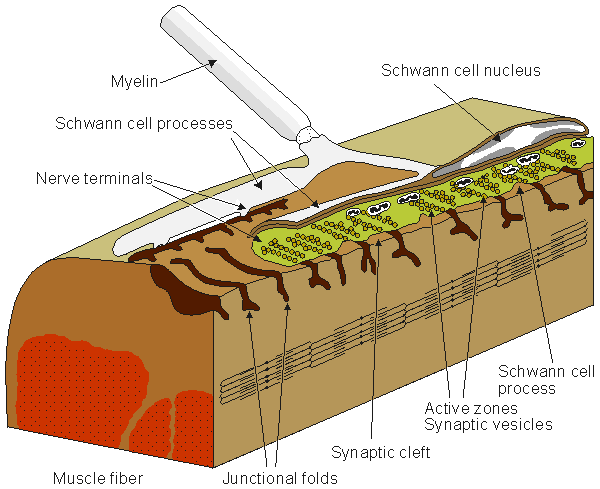
Fig. 5.1. The neuromuscular (synaptic) junction. Many features of this junction are also seen in the nerve-nerve synapse. The terminal ending of the prejunctional cell contains many vesicles, which are packages of the neurotransmitter acetylcholine (ACh). The gap between the pre- and postjunctional membrane is on the order of 15-30 nm. The transmitter is released by the arrival of an action impulse in the nerve; it diffuses and binds to receptors on the postjunctional muscle membrane, bringing about an EPSP and the initiation of a muscle action potential.
5.2.2 Excitatory and Inhibitory Synapses
In the neuromuscular junction, upon arrival of an action pulse at the motor neuron ending, acetylcholine (ACh) is released into the cleft. It diffuses across the gap to the muscle membrane where it binds to specialized receptors, resulting in a simultaneous increase in membrane permeability to both sodium and potassium ions. Because the relative effect on sodium exceeds that of potassium (described quantitatively later in this section), the membrane depolarizes and a postsynaptic action potential results. The process is always excitatory. Furthermore, arrival of a single action potential at the prejunctional site always results in sufficient release of transmitter to produce a transthreshold depolarization and initiate an action potential in the muscle.
 Synaptic inhibition occurs at nerve-nerve (neuro-neuro) junctions when presynaptic activity releases a transmitter that hyperpolarizes the postsynaptic membrane (i.e., makes its membrane voltage more negative). In theory, hyperpolarization could result from elevation of either potassium or chloride permeability because the equilibrium potential of each is more negative than the normal resting potential (which is influenced in the positive direction by the presence of sodium). In actuality, however, inhibition is due to elevated chloride permeability.
Synaptic inhibition occurs at nerve-nerve (neuro-neuro) junctions when presynaptic activity releases a transmitter that hyperpolarizes the postsynaptic membrane (i.e., makes its membrane voltage more negative). In theory, hyperpolarization could result from elevation of either potassium or chloride permeability because the equilibrium potential of each is more negative than the normal resting potential (which is influenced in the positive direction by the presence of sodium). In actuality, however, inhibition is due to elevated chloride permeability.
 In contrast with the neuromuscular (neuro-myo) junction, a single excitatory input to a neuro-neuro synapse is completely inadequate to depolarize the postjunctional membrane to threshold. In fact, with perhaps thousands of both excitatory and inhibitory inputs on the postjunctional cell, a spatial and temporal summation is continually taking place, and the membrane voltage will fluctuate. When, finally, a threshold of perhaps 10-15 mV is reached, an action potential results. In this way, an important integrative process takes place at the inputs to each nerve cell. The reader with computer science experience can appreciate the tremendous possibilities for information processing that can (and do!) take place, particularly when one considers that there are perhaps 1012 neurons and 1015 synapses in the human brain. This is indeed a neural net.
In contrast with the neuromuscular (neuro-myo) junction, a single excitatory input to a neuro-neuro synapse is completely inadequate to depolarize the postjunctional membrane to threshold. In fact, with perhaps thousands of both excitatory and inhibitory inputs on the postjunctional cell, a spatial and temporal summation is continually taking place, and the membrane voltage will fluctuate. When, finally, a threshold of perhaps 10-15 mV is reached, an action potential results. In this way, an important integrative process takes place at the inputs to each nerve cell. The reader with computer science experience can appreciate the tremendous possibilities for information processing that can (and do!) take place, particularly when one considers that there are perhaps 1012 neurons and 1015 synapses in the human brain. This is indeed a neural net.
 Presynaptic inhibition is another inhibition mechanism. In this case an inhibitory nerve ending (from another axon known as the presynaptic inhibitor) is synapsed to an excitatory presynaptic terminal. The inhibitory nerve releases a transmitter that partially depolarizes the presynaptic cell. As a consequence, activation arising in the presynaptic fiber is diminished, hence the release of transmitter is reduced. As a result, the degree of excitation produced in the postsynaptic cell is reduced (hence an inhibitory effect).
Presynaptic inhibition is another inhibition mechanism. In this case an inhibitory nerve ending (from another axon known as the presynaptic inhibitor) is synapsed to an excitatory presynaptic terminal. The inhibitory nerve releases a transmitter that partially depolarizes the presynaptic cell. As a consequence, activation arising in the presynaptic fiber is diminished, hence the release of transmitter is reduced. As a result, the degree of excitation produced in the postsynaptic cell is reduced (hence an inhibitory effect).
 The falling phase of the EPSP is characterized by a single time constant - that is, the time required for the response to a single excitatory stimulus to diminish to 1/e of its maximum. This is an important value. If a sequence of afferent stimuli occurs in a very short time interval, then temporal summation of the EPSPs occurs, yielding a growing potential. Similarly, if activity occurs at more than one synaptic knob simultaneously (or within the length of the aforementioned time constant), then spatial summation results. The additive effect on a synapse is nonlinear. Furthermore, the individual synapses interact in an extremely complicated way (Stevens, 1968). Despite these complexities, it has been shown experimentally that both spatial and temporal summation generally behave in a simple linear manner (Granit, Haase, and Rutledge, 1960; Granit and Renkin, 1961).
The falling phase of the EPSP is characterized by a single time constant - that is, the time required for the response to a single excitatory stimulus to diminish to 1/e of its maximum. This is an important value. If a sequence of afferent stimuli occurs in a very short time interval, then temporal summation of the EPSPs occurs, yielding a growing potential. Similarly, if activity occurs at more than one synaptic knob simultaneously (or within the length of the aforementioned time constant), then spatial summation results. The additive effect on a synapse is nonlinear. Furthermore, the individual synapses interact in an extremely complicated way (Stevens, 1968). Despite these complexities, it has been shown experimentally that both spatial and temporal summation generally behave in a simple linear manner (Granit, Haase, and Rutledge, 1960; Granit and Renkin, 1961).
 Synaptic transmission has been compared to an electric information transfer circuit in the following way: In the nerve axon the information is transferred by means of nerve impulses in "digital" or, more accurately, "pulse-code modulated" form. In the synapse, information is conducted with the transmitter substance in analog form, to be converted again in the next neuron into "digital" form. Though this analogy is not correct in all aspects, it illustrates the character of the neural information chain.
Synaptic transmission has been compared to an electric information transfer circuit in the following way: In the nerve axon the information is transferred by means of nerve impulses in "digital" or, more accurately, "pulse-code modulated" form. In the synapse, information is conducted with the transmitter substance in analog form, to be converted again in the next neuron into "digital" form. Though this analogy is not correct in all aspects, it illustrates the character of the neural information chain.
5.2.3 Reflex Arc
The driver of a car receives visual signals via photoreceptors that initiate coded afferent impulses that ascend nerve fibers and terminate in the visual cortex. Once the brain has processed the information, it sends efferent signals to the muscles in the foot and hands. Thus the car is slowed down and can make a right turn. But if our hand is mistakenly brought to rest on a hot surface, a set of signals to the hand and arm muscles result that are not initiated in the higher centers; cognition comes into play only after the fact. We say that a reflex path is involved in both of these examples. The first is complex and involves higher centers in the central nervous system, whereas the second describes a simpler reflex at a lower level. In fact, a great deal of reflex activity is taking place at all times of which we are unaware. For example, input signals are derived from internal sensors, such as blood pressure, or oxygen saturation in the blood, and so on, leading to an adjustment of heart rate, breathing rate, etc.
 The reflex arc, illustrated above, is considered to be the basic unit of integrated neural activity. It consists essentially of a sensory receptor, an afferent neuron, one or more synapses, an efferent neuron, and a muscle or other effector. The connection between afferent and efferent pathways is found, generally, in the spinal cord or the brain. The simplest reflex involves only a single synapse between afferent and efferent neurons (a monosynaptic reflex); an example is the familiar knee jerk reflex.
The reflex arc, illustrated above, is considered to be the basic unit of integrated neural activity. It consists essentially of a sensory receptor, an afferent neuron, one or more synapses, an efferent neuron, and a muscle or other effector. The connection between afferent and efferent pathways is found, generally, in the spinal cord or the brain. The simplest reflex involves only a single synapse between afferent and efferent neurons (a monosynaptic reflex); an example is the familiar knee jerk reflex.
 Homeostasis refers to the various regulatory processes in the body that maintain a normal state in the face of disturbances. The autonomic nervous system is organized to accomplish this automatically with regard to many organs of the body; its activity, like that of the somatic nervous system, is based on the reflex arc. In this case signals, which arise at visceral receptors, are conveyed via afferent neurons to the central nervous system, where integration takes place, resulting in efferent signals to visceral effectors (in particular, smooth muscle) to restore or maintain normal conditions. Integration of signals affecting blood pressure and respiration takes place in the medulla oblongata; those controlling pupillary response to light are integrated in the midbrain, whereas those responding to body temperature are integrated in the hypothalamus - to give only a few examples.
Homeostasis refers to the various regulatory processes in the body that maintain a normal state in the face of disturbances. The autonomic nervous system is organized to accomplish this automatically with regard to many organs of the body; its activity, like that of the somatic nervous system, is based on the reflex arc. In this case signals, which arise at visceral receptors, are conveyed via afferent neurons to the central nervous system, where integration takes place, resulting in efferent signals to visceral effectors (in particular, smooth muscle) to restore or maintain normal conditions. Integration of signals affecting blood pressure and respiration takes place in the medulla oblongata; those controlling pupillary response to light are integrated in the midbrain, whereas those responding to body temperature are integrated in the hypothalamus - to give only a few examples.
5.2.4 Electric Model of the Synapse
At the neuromuscular junction, Fatt and Katz (1951) showed that acetylcholine significantly increases the permeability of the cell membrane to small ions, whereas Takeuchi and Takeuchi (1960) demonstrated that chloride conductance was unaffected (in fact, gCl 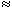 0). What happens if the membrane becomes equally permeable to sodium and potassium ions? Such a condition would alter the membrane potential from near the potassium Nernst potential to a value that approximates the average of the sodium and potassium equilibrium potentials. (This potential, in turn, is close to zero transmembrane voltage and is entirely adequate to initiate an activation.) If the postsynaptic region is voltage-clamped, the value that reduces the membrane current to zero during transmitter release is called the reversal voltage Vr. One can show that it equals the average Nernst potential of sodium and potassium, as mentioned above. In the neuromuscular junction in skeletal muscle, this reversal voltage is about -15 mV.
0). What happens if the membrane becomes equally permeable to sodium and potassium ions? Such a condition would alter the membrane potential from near the potassium Nernst potential to a value that approximates the average of the sodium and potassium equilibrium potentials. (This potential, in turn, is close to zero transmembrane voltage and is entirely adequate to initiate an activation.) If the postsynaptic region is voltage-clamped, the value that reduces the membrane current to zero during transmitter release is called the reversal voltage Vr. One can show that it equals the average Nernst potential of sodium and potassium, as mentioned above. In the neuromuscular junction in skeletal muscle, this reversal voltage is about -15 mV.
 The electric behavior at a synapse can be estimated by examining an equivalent circuit of the postsynaptic membrane, such as that shown in Figure 5.2. Two regions are identified: One represents the membrane associated with receptors sensitive to the transmitter, and the other the normal excitable membrane of the cell. In Figure 5.2 these two regions are represented by discrete elements, but in reality these are distributed along the structure that constitutes the actual cell. This figure depicts a neuromuscular junction, where the release of acetylcholine results in the elevation of sodium and potassium conductance in the target region, which is in turn depicted by the closing of the ACh switch. Upon closure of this switch,
The electric behavior at a synapse can be estimated by examining an equivalent circuit of the postsynaptic membrane, such as that shown in Figure 5.2. Two regions are identified: One represents the membrane associated with receptors sensitive to the transmitter, and the other the normal excitable membrane of the cell. In Figure 5.2 these two regions are represented by discrete elements, but in reality these are distributed along the structure that constitutes the actual cell. This figure depicts a neuromuscular junction, where the release of acetylcholine results in the elevation of sodium and potassium conductance in the target region, which is in turn depicted by the closing of the ACh switch. Upon closure of this switch,
 DINa = DGNa(Vm - VNa) DINa = DGNa(Vm - VNa) | (5.1) |
 DIK = DGK(Vm - VK) DIK = DGK(Vm - VK) | (5.2) |
| where | INa, IK | = | sodium and potassium ion currents [µA/cm²] |
| | DGNa, DGK | = | additional sodium and potassium conductances following activation by ACh (i.e., nearly equal large conductances) [mS/cm²] |
| | VNa, VK | = | the Nernst voltages corresponding to the sodium and potassium concentrations [mV] |
| | Vm | = | membrane voltage [mV] |
Fig. 5.2. (A) Electric model of the postsynaptic cell with excitatory synapse (a neuromuscular junction is specifically represented). Most of the cell is bounded by normal excitable membrane, as described on the left. In addition, a specialized postsynaptic region (end-plate) exists that is sensitive to the chemical transmitter ACh. When the ACh is released, it diffuses to receptor sites on the postjunctional membrane, resulting in the opening of potassium and sodium gates. This effect is mimicked in the model through closing of the switch, hence introducing the high transmembrane potassium and sodium conductance (DGNa and DGK).
(B) The corresponding model with an inhibitory synapse.
If we now introduce and maintain the reversal voltage across the postsynaptic membrane through a voltage clamp, Equations 5.1 and 5.2 are replaced by:
 DINa = DGNa(VR - VNa) DINa = DGNa(VR - VNa) | (5.3) |
 DIK = DGK(VR - VK) DIK = DGK(VR - VK) | (5.4) |
since the transmembrane voltage Vm takes the value VR, the reversal voltage.
 For the conditions described by Equations 5.3 and 5.4, since the total current at the reversal voltage is zero, it follows that the sodium and potassium ion currents are equal and opposite in sign (i.e., DINa = -DIK). Consequently, applying this condition to Equations 5.3 and 5.4 results in the following:
For the conditions described by Equations 5.3 and 5.4, since the total current at the reversal voltage is zero, it follows that the sodium and potassium ion currents are equal and opposite in sign (i.e., DINa = -DIK). Consequently, applying this condition to Equations 5.3 and 5.4 results in the following:
 DGNa(VR - VNa) = - DGK(VR - VK) DGNa(VR - VNa) = - DGK(VR - VK) | (5.5) |
Collecting terms in Equation 5.5 gives
 (DGNa + DGK) VR = DGNaVNa - DGKVK (DGNa + DGK) VR = DGNaVNa - DGKVK | (5.6) |
and solving for the reversal voltage results in
 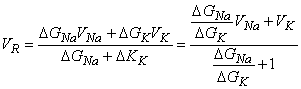 | (5.7) |
 From Equation 5.7 it is easy to see that if the introduction of ACh causes an equal increase in the sodium and potassium conductances - that is, if
From Equation 5.7 it is easy to see that if the introduction of ACh causes an equal increase in the sodium and potassium conductances - that is, if
 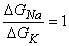 | (5.8) |
then
 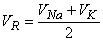 | (5.9) |
as noted previously. For the frog's neuromuscular junction the reversal voltage comes to around -25 mV. In practice, the reversal voltage is a little closer to zero, which means that ACh increases the sodium conductance a little more than it does the potassium conductance. It is also clear that the increase of these sodium and potassium conductances must occur simultaneously. The differences in the mechanisms of the membrane activation and synaptic voltages are described in Table 5.1.
Table 5.1. Comparison of the mechanisms of membrane activation with synaptic voltage change for the
post-synaptic neuromuscular junction.
Feature
|
Membrane region
|
Synaptic region
|
|
| Early effect |
depolarization |
arrival of acetylcholine |
Changes in membrane
conductance during |
|
|
 - rising phase - rising phase |
specific increase in GNa
|
simultaneous increase in GNa
and GK
|
 - falling phase - falling phase |
specific increase in GK |
passive decay |
Equilibrium voltage
of active membrane |
VNa = +50 mV |
reversal voltage close to 0 mV |
| Other features |
regenerative ascent followed by refractory period |
no evidence for regenerative action or refractoriness |
| Pharmacology |
blocked by TTX, not influenced by curare |
blocked by curare, not influenced by TTX |
|
| Source: After Kuffler, Nicholls and Martin, 1984. |
 Returning to Figure 5.2, and applying Thevenin's theorem, we can simplify the receptor circuit to consist of a single battery whose emf is the average of VNa and VK (hence VR), and with a conductivity gR = gNa + gK. Its effect on the normal membrane of the postsynaptic cell can be calculated since the total current at any node is necessarily zero - that is, there are no applied currents. Consequently,
Returning to Figure 5.2, and applying Thevenin's theorem, we can simplify the receptor circuit to consist of a single battery whose emf is the average of VNa and VK (hence VR), and with a conductivity gR = gNa + gK. Its effect on the normal membrane of the postsynaptic cell can be calculated since the total current at any node is necessarily zero - that is, there are no applied currents. Consequently,
 GR (Vm - VR) + GK(Vm - VK) + GNa(Vm - VNa) = 0 GR (Vm - VR) + GK(Vm - VK) + GNa(Vm - VNa) = 0 | (5.10) |
The chloride path in Figure 5.2 is not included in Equation 5.10, since gCl 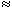 0 , as noted above. Solving for the postsynaptic potential Vm results in
0 , as noted above. Solving for the postsynaptic potential Vm results in
 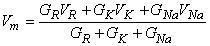 | (5.11) |
This expression is only approximate since the distributed membrane is represented by a discrete (lumped) membrane. In addition, if the membrane is brought to or beyond threshold, then the linear circuit representation of Figure 5.2 becomes invalid. Nevertheless, Equation 5.11 should be a useful measure of whether the postsynaptic potential is likely to result in excitation of the postsynaptic cell.
5.3 RECEPTOR CELLS
5.3.1 Introduction
To begin the overview of the nervous system, we consider the sensory inputs to the body and how they are initiated. There are many specialized receptor cells, each characterized by a modality to which it is particularly sensitive and to which it responds by generating a train of action pulses. We are particularly interested in the structure and function of these receptor cells and focus on the Pacinian corpuscle as an example.
5.3.2 Various Types of Receptor Cells
One of the most important properties required to maintain the life of the living organism is the ability to react to external stimuli. Sense organs are specialized for this task. The essential element of these organs is the receptor cell, which responds to physical and chemical stimuli by sending information to the central nervous system. In general, a receptor cell may respond to several forms of energy, but each is specialized to respond primarily to one particular type. For instance, the rods and cones in the eye (photoreceptors) can respond to pressure, but they have a particularly low threshold to electromagnetic energy in the certain frequency band of electromagnetic radiation, namely visible light. In fact, they are the only receptor cells with such low thresholds to light stimulus.
 There are at least a dozen conscious sense modalities with which we are familiar. In addition, there are other sensory receptors whose information processing goes on without our awareness. Together these may be classified as (1) extroreceptors, which sense stimuli arising external to the body; (2) introreceptors, which respond to physical or chemical qualities within the body; and (3) proprioceptors, which provide information on the body's position. Examples in each of these categories include the following:
There are at least a dozen conscious sense modalities with which we are familiar. In addition, there are other sensory receptors whose information processing goes on without our awareness. Together these may be classified as (1) extroreceptors, which sense stimuli arising external to the body; (2) introreceptors, which respond to physical or chemical qualities within the body; and (3) proprioceptors, which provide information on the body's position. Examples in each of these categories include the following:
- Extroreceptors
- Photoreceptors in the retina for, vision
- Chemoreceptors for sensing of smell and taste
- Mechanoreceptors for sensing sound, in the cochlea, or in the skin, for touch sensation
- Thermoreceptors (i.e., Krause and Ruffini cells), for sensing cold and heat
- Introreceptors
- Chemoreceptors in the carotid artery and aorta, responding to the partial pressure of oxygen, and in the breathing center, responding to the partial pressure of carbon dioxide
- Mechanoreceptors in the labyrinth
- Osmoreceptors in the hypothalamus, registering the osmotic pressure of the blood
- Proprioceptors
- Muscle spindle, responding to changes in muscle length
- Golgi tendon organ, measuring muscle tension
 The sensory receptor contains membrane regions that respond to one of the various forms of incident stimuli by a depolarization (or hyperpolarization). In some cases the receptor is actually part of the afferent neuron but, in others it consists of a separate specialized cell. All receptor cells have a common feature: They are transducers - that is, they change energy from one form to another. For instance, the sense of touch in the skin arises from the conversion of mechanical and/or thermal energy into the electric energy (ionic currents) of the nerve impulse. In general, the receptor cells do not generate an activation impulse themselves. Instead, they generate a gradually increasing potential, which triggers activation of the afferent nerve fiber to which they are connected.
The sensory receptor contains membrane regions that respond to one of the various forms of incident stimuli by a depolarization (or hyperpolarization). In some cases the receptor is actually part of the afferent neuron but, in others it consists of a separate specialized cell. All receptor cells have a common feature: They are transducers - that is, they change energy from one form to another. For instance, the sense of touch in the skin arises from the conversion of mechanical and/or thermal energy into the electric energy (ionic currents) of the nerve impulse. In general, the receptor cells do not generate an activation impulse themselves. Instead, they generate a gradually increasing potential, which triggers activation of the afferent nerve fiber to which they are connected.
 The electric events in receptors may be separated into two distinct components:
The electric events in receptors may be separated into two distinct components:
- Development of a receptor voltage, which is the graded response of the receptor to the stimulus. It is the initial electric event in the receptor.
- Subsequent buildup of a generator voltage, which is the electric phenomenon that triggers impulse propagation in the axon. It is the final electric event before activation, which, in turn, follows the "all-or-nothing" law.
 These voltage changes are, however, one and the same in a receptor such as the Pacinian corpuscle, in which there are no specialized receptor cells. But in cases like the retina where specialized receptor cells (i.e., the rods and cones) do exist, these voltages are separate. In the following, we consider the Pacinian corpuscle in more detail (Granit, 1955).
These voltage changes are, however, one and the same in a receptor such as the Pacinian corpuscle, in which there are no specialized receptor cells. But in cases like the retina where specialized receptor cells (i.e., the rods and cones) do exist, these voltages are separate. In the following, we consider the Pacinian corpuscle in more detail (Granit, 1955).
 Because the neural output is carried in the form of all-or-nothing action pulses, we must look to another form of signal than one that is amplitude modulated. In fact, the generator or receptor potentials cause repetitive firing of action pulses on the afferent neuron, and the firing rate (and rate of change) is reflective of the sensory input. This coded signal can be characteristic of the modality being transduced.
Because the neural output is carried in the form of all-or-nothing action pulses, we must look to another form of signal than one that is amplitude modulated. In fact, the generator or receptor potentials cause repetitive firing of action pulses on the afferent neuron, and the firing rate (and rate of change) is reflective of the sensory input. This coded signal can be characteristic of the modality being transduced.
 In a process of adaptation, the frequency of action potential firing decreases in time with respect to a steady stimulus. One can separate the responses into fast and slow rates of adaptation, depending on how quickly the frequency reduction takes place (i.e., muscle spindle is slow whereas touch is fast).
In a process of adaptation, the frequency of action potential firing decreases in time with respect to a steady stimulus. One can separate the responses into fast and slow rates of adaptation, depending on how quickly the frequency reduction takes place (i.e., muscle spindle is slow whereas touch is fast).
5.3.3 The Pacinian Corpuscle
The Pacinian corpuscle is a touch receptor which, under the microscope, resembles an onion (see Figure 5.3). It is 0.5-1 mm long and 0.3-0.7 mm thick and consists of several concentric layers. The center of the corpuscle includes the core, where the unmyelinated terminal part of the afferent neuron is located. The first node of Ranvier is also located inside the core. Several mitochondria exist in the corpuscle, indicative of high energy production.
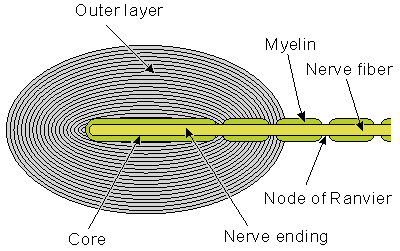
Fig. 5.3. The Pacinian corpuscle consists of a myelinated sensory neuron whose terminal portion is unmyelinated. The unmyelinated nerve ending and the first node lie within a connective tissue capsule, as shown.
 Werner R. Loewenstein (1959) stimulated the corpuscle with a piezoelectric crystal and measured the generator voltage (from the unmyelinated terminal axon) and the action potential (from the nodes of Ranvier) with an external electrode. He peeled off the layers of the corpuscle, and even after the last layer was removed, the corpuscle generated signals similar to those observed with the capsule intact (see recordings shown in Figure 5.4).
Werner R. Loewenstein (1959) stimulated the corpuscle with a piezoelectric crystal and measured the generator voltage (from the unmyelinated terminal axon) and the action potential (from the nodes of Ranvier) with an external electrode. He peeled off the layers of the corpuscle, and even after the last layer was removed, the corpuscle generated signals similar to those observed with the capsule intact (see recordings shown in Figure 5.4).
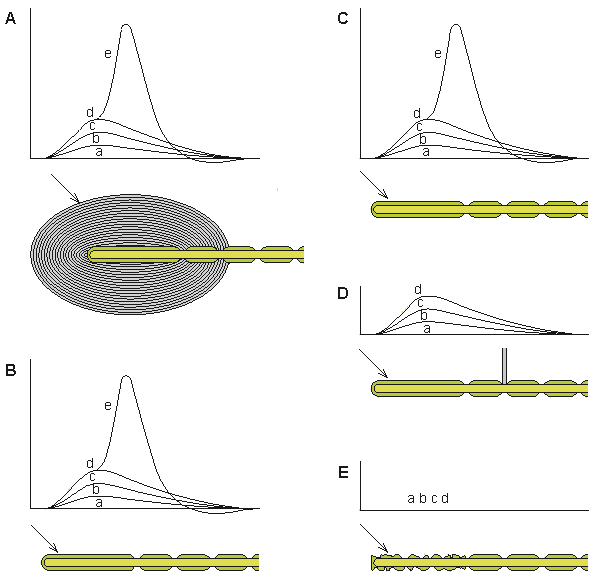
 The generator voltage has properties similar to these of the excitatory postsynaptic voltage. (The generator voltage is a graded response whereby a weak stimulus generates a low generator voltage whereas a strong stimulus generates a large generator voltage.) Even partial destruction of the corpuscle did not prevent it from producing a generator voltage. But when Loewenstein destroyed the nerve ending itself, a generator voltage could no longer be elicited. This observation formed the basis for supposing that the transducer itself was located in the nerve ending. The generator voltage does not propagate on the nerve fiber (in fact, the nerve ending is electrically inexcitable) but, rather, triggers the activation process in the first node of Ranvier by electrotonic (passive) conduction. If the first node is blocked, no activation is initiated in the nerve fiber.
The generator voltage has properties similar to these of the excitatory postsynaptic voltage. (The generator voltage is a graded response whereby a weak stimulus generates a low generator voltage whereas a strong stimulus generates a large generator voltage.) Even partial destruction of the corpuscle did not prevent it from producing a generator voltage. But when Loewenstein destroyed the nerve ending itself, a generator voltage could no longer be elicited. This observation formed the basis for supposing that the transducer itself was located in the nerve ending. The generator voltage does not propagate on the nerve fiber (in fact, the nerve ending is electrically inexcitable) but, rather, triggers the activation process in the first node of Ranvier by electrotonic (passive) conduction. If the first node is blocked, no activation is initiated in the nerve fiber.
 The ionic flow mechanism underlying the generator (receptor) voltage is the same as that for the excitatory postsynaptic voltage. Thus deformation of the Pacinian corpuscle increases both the sodium and potassium conductances such that their ratio (PNa/PK) increases and depolarization of the membrane potential results. As a result, the following behavior is observed:
The ionic flow mechanism underlying the generator (receptor) voltage is the same as that for the excitatory postsynaptic voltage. Thus deformation of the Pacinian corpuscle increases both the sodium and potassium conductances such that their ratio (PNa/PK) increases and depolarization of the membrane potential results. As a result, the following behavior is observed:
- Small (electrotonic) currents flow from the depolarized unmyelinated region of the axon to the nodes of Ranvier.
- On the unmyelinated membrane, local graded generator voltages are produced independently at separate sites.
- The aforementioned separate receptor voltages are summed in the first node of Ranvier.
- The summed receptor voltages, which exceed threshold at the first node of Ranvier, generate an action impulse. This is evidence of spatial summation, and is similar to the same phenomenon observed in the excitatory postsynaptic potential.
5.4 ANATOMY AND PHYSIOLOGY OF THE BRAIN
5.4.1 Introduction
Action pulses generated at the distal end of sensory neurons propagate first to the cell body and then onward, conveyed by long axonal pathways. These ascend the spinal cord (dorsal root) until they reach the lower part of the central nervous system. Here the signals are relayed to other neurons, which in turn relay them onward. Three or four such relays take place before the signals reach particular loci in the cerebral cortex. Signal processing takes place at all levels, resulting in the state of awareness and conscious recognition of the various signals that characterize human physiology. The important integrative activity of the brain has been the subject of intense study, but its complexity has slowed the rate of progress. In this section a brief description is given of both the anatomy and the physiology of the brain.
5.4.2 Brain Anatomy
The brain consists of 1010-1011 neurons that are very closely interconnected via axons and dendrites. The neurons themselves are vastly outnumbered by glial cells. One neuron may receive stimuli through synapses from as many as 103 to 105 other neurons (Nunez, 1981). Embryologically the brain is formed when the front end of the central neural system has folded. The brain consists of five main parts, as described in Figure 5.5:
- The cerebrum, including the two cerebral hemispheres
- The interbrain (diencephalon)
- The midbrain
- The pons Varolii and cerebellum
- The medulla oblongata
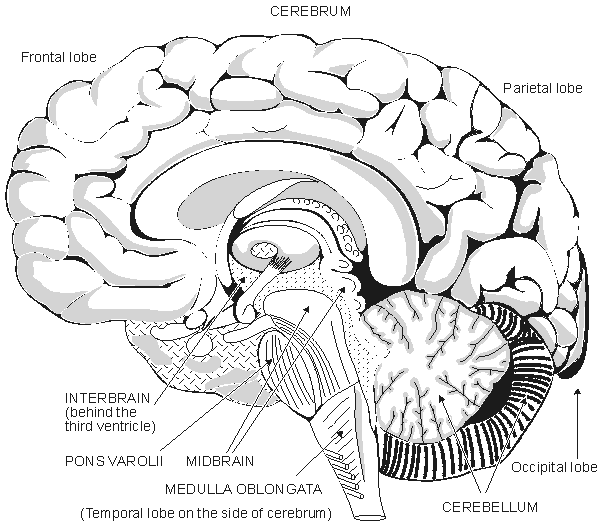
Fig. 5.5. The anatomy of the brain.
 The entire human brain weighs about 1500 g (Williams and Warwick, 1989). In the brain the cerebrum is the largest part. The surface of the cerebrum is strongly folded. These folds are divided into two hemispheres which are separated by a deep fissure and connected by the corpus callosum. Existing within the brain are three ventricles containing cerebrospinal fluid. The hemispheres are divided into the following lobes: lobus frontalis, lobus parietalis, lobus occipitalis, and lobus temporalis. The surface area of the cerebrum is about 1600 cm², and its thickness is 3 mm. Six layers, or laminae, each consisting of different neuronal types and populations, can be observed in this surface layer. The higher cerebral functions, accurate sensations, and the voluntary motor control of muscles are located in this region.
The entire human brain weighs about 1500 g (Williams and Warwick, 1989). In the brain the cerebrum is the largest part. The surface of the cerebrum is strongly folded. These folds are divided into two hemispheres which are separated by a deep fissure and connected by the corpus callosum. Existing within the brain are three ventricles containing cerebrospinal fluid. The hemispheres are divided into the following lobes: lobus frontalis, lobus parietalis, lobus occipitalis, and lobus temporalis. The surface area of the cerebrum is about 1600 cm², and its thickness is 3 mm. Six layers, or laminae, each consisting of different neuronal types and populations, can be observed in this surface layer. The higher cerebral functions, accurate sensations, and the voluntary motor control of muscles are located in this region.
 The interbrain or diencephalon is surrounded by the cerebrum and is located around the third ventricle. It includes the thalamus, which is a bridge connecting the sensory paths. The hypothalamus, which is located in the lower part of the interbrain, is important for the regulation of autonomic (involuntary) functions. Together with the hypophysis, it regulates hormonal secretions. The midbrain is a small part of the brain. The pons Varolii is an interconnection of neural tracts; the cerebellum controls fine movement. The medulla oblongata resembles the spinal cord to which it is immediately connected. Many reflex centers, such as the vasomotor center and the breathing center, are located in the medulla oblongata.
The interbrain or diencephalon is surrounded by the cerebrum and is located around the third ventricle. It includes the thalamus, which is a bridge connecting the sensory paths. The hypothalamus, which is located in the lower part of the interbrain, is important for the regulation of autonomic (involuntary) functions. Together with the hypophysis, it regulates hormonal secretions. The midbrain is a small part of the brain. The pons Varolii is an interconnection of neural tracts; the cerebellum controls fine movement. The medulla oblongata resembles the spinal cord to which it is immediately connected. Many reflex centers, such as the vasomotor center and the breathing center, are located in the medulla oblongata.
 In the cerebral cortex one may locate many different areas of specialized brain function (Penfield and Rasmussen, 1950; Kiloh, McComas, and Osselton, 1981). The higher brain functions occur in the frontal lobe, the visual center is located in the occipital lobe, and the sensory area and motor area are located on both sides of the central fissure. There are specific areas in the sensory and motor cortex whose elements correspond to certain parts of the body. The size of each such area is proportional to the required accuracy of sensory or motor control. These regions are described in Figure 5.6. Typically, the sensory areas represented by the lips and the hands are large, and the areas represented by the midbody and eyes are small. The visual center is located in a different part of the brain. The motor area, the area represented by the hands and the speaking organs, is large.
In the cerebral cortex one may locate many different areas of specialized brain function (Penfield and Rasmussen, 1950; Kiloh, McComas, and Osselton, 1981). The higher brain functions occur in the frontal lobe, the visual center is located in the occipital lobe, and the sensory area and motor area are located on both sides of the central fissure. There are specific areas in the sensory and motor cortex whose elements correspond to certain parts of the body. The size of each such area is proportional to the required accuracy of sensory or motor control. These regions are described in Figure 5.6. Typically, the sensory areas represented by the lips and the hands are large, and the areas represented by the midbody and eyes are small. The visual center is located in a different part of the brain. The motor area, the area represented by the hands and the speaking organs, is large.
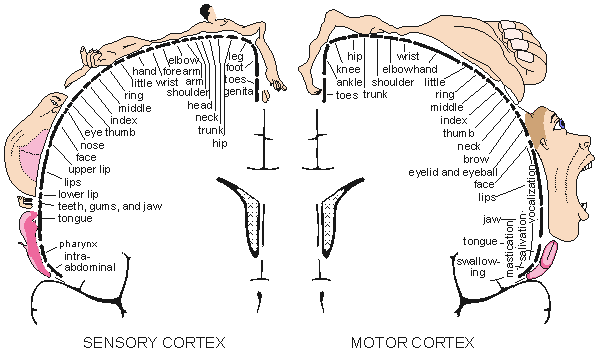
Fig. 5.6. The division of sensory (left) and motor (right) functions in the cerebral cortex. (From Penfield and Rasmussen, 1950.)
5.4.3 Brain Function
Most of the information from the sensory organs is communicated through the spinal cord to the brain. There are special tracts in both spinal cord and brain for various modalities. For example, touch receptors in the trunk synapse with interneurons in the dorsal horn of the spinal cord. These interneurons (sometimes referred to as second sensory neurons) then usually cross to the other side of the spinal cord and ascend the white matter of the cord to the brain in the lateral spinothalamic tract. In the brain they synapse again with a second group of interneurons (or third sensory neuron) in the thalamus. The third sensory neurons connect to higher centers in the cerebral cortex.
 In the area of vision, afferent fibers from the photoreceptors carry signals to the brain stem through the optic nerve and optic tract to synapse in the lateral geniculate body (a part of the thalamus). From here axons pass to the occipital lobe of the cerebral cortex. In addition, branches of the axons of the optic tract synapse with neurons in the zone between thalamus and midbrain which is the pretectal nucleus and superior colliculus. These, in turn, synapse with preganglionic parasympathetic neurons whose axons follow the oculomotor nerve to the ciliary ganglion (located just behind the eyeball). The reflex loop is closed by postganglionic fibers which pass along ciliary nerves to the iris muscles (controlling pupil aperture) and to muscles controlling the lens curvature (adjusting its refractive or focusing qualities). Other reflexes concerned with head and/or eye movements may also be initiated.
In the area of vision, afferent fibers from the photoreceptors carry signals to the brain stem through the optic nerve and optic tract to synapse in the lateral geniculate body (a part of the thalamus). From here axons pass to the occipital lobe of the cerebral cortex. In addition, branches of the axons of the optic tract synapse with neurons in the zone between thalamus and midbrain which is the pretectal nucleus and superior colliculus. These, in turn, synapse with preganglionic parasympathetic neurons whose axons follow the oculomotor nerve to the ciliary ganglion (located just behind the eyeball). The reflex loop is closed by postganglionic fibers which pass along ciliary nerves to the iris muscles (controlling pupil aperture) and to muscles controlling the lens curvature (adjusting its refractive or focusing qualities). Other reflexes concerned with head and/or eye movements may also be initiated.
 Motor signals to muscles of the trunk and periphery from higher motor centers of the cerebral cortex first travel along upper motor neurons to the medulla oblongata. From here most of the axons of the upper motor neurons cross to the other side of the central nervous system and descend the spinal cord in the lateral corticospinal tract; the remainder travel down the cord in the anterior corticospinal tract. The upper motor neurons eventually synapse with lower motor neurons in the ventral horn of the spinal cord; the lower motor neurons complete the path to the target muscles. Most reflex motor movements involve complex neural integration and coordinate signals to the muscles involved in order to achieve a smooth performance.
Motor signals to muscles of the trunk and periphery from higher motor centers of the cerebral cortex first travel along upper motor neurons to the medulla oblongata. From here most of the axons of the upper motor neurons cross to the other side of the central nervous system and descend the spinal cord in the lateral corticospinal tract; the remainder travel down the cord in the anterior corticospinal tract. The upper motor neurons eventually synapse with lower motor neurons in the ventral horn of the spinal cord; the lower motor neurons complete the path to the target muscles. Most reflex motor movements involve complex neural integration and coordinate signals to the muscles involved in order to achieve a smooth performance.
 Effective integration of sensory information requires that this information be collected at a single center. In the cerebral cortex, one can indeed locate specific areas identified with specific sensory inputs (Penfield and Rasmussen, 1950; Kiloh, McComas, and Osselton, 1981). While the afferent signals convey information regarding stimulus strength, recognition of the modality depends on pinpointing the anatomical classification of the afferent pathways. (This can be demonstrated by interchanging the afferent fibers from, say, auditory and tactile receptors, in which case sound inputs are perceived as of tactile origin and vice versa.)
Effective integration of sensory information requires that this information be collected at a single center. In the cerebral cortex, one can indeed locate specific areas identified with specific sensory inputs (Penfield and Rasmussen, 1950; Kiloh, McComas, and Osselton, 1981). While the afferent signals convey information regarding stimulus strength, recognition of the modality depends on pinpointing the anatomical classification of the afferent pathways. (This can be demonstrated by interchanging the afferent fibers from, say, auditory and tactile receptors, in which case sound inputs are perceived as of tactile origin and vice versa.)
 The higher brain functions take place in the frontal lobe, the visual center is in the occipital lobe, the sensory area and motor area are located on both sides of the central fissure. As described above, there is an area in the sensory cortex whose elements correspond to each part of the body. In a similar way, a part of the brain contains centers for generating command (efferent) signals for control of the body's musculature. Here, too, one finds projections from specific cortical areas to specific parts of the body.
The higher brain functions take place in the frontal lobe, the visual center is in the occipital lobe, the sensory area and motor area are located on both sides of the central fissure. As described above, there is an area in the sensory cortex whose elements correspond to each part of the body. In a similar way, a part of the brain contains centers for generating command (efferent) signals for control of the body's musculature. Here, too, one finds projections from specific cortical areas to specific parts of the body.
5.5 CRANIAL NERVES
In the central nervous system there are 12 cranial nerves. They leave directly from the cranium rather than the spinal cord. They are listed in Table 5.2 along with their functions. The following cranial nerves have special importance: the olfactory (I) and optic (II) nerves, which carry sensory information from the nose and eye; and the auditory-vestibular (VIII) nerve, which carries information from the ear and the balance organ. Sensory information from the skin of the face and head is carried by the trigeminal (V) nerve. Eye movements are controlled by three cranial nerves (III, IV, and VI). The vagus nerve (X) controls heart function and internal organs as well as blood vessels.
Table 5.2. The cranial nerves
| |
Number |
Name |
Sensory/
Motor |
Functions |
Origin or terminus
in the brain |
|
| |
I |
olfactory |
s |
smell |
cerebral hemispheres
(ventral part) |
| |
II |
optic |
s |
vision |
thalamus |
| |
III |
oculomotor |
m |
eye movement |
midbrain |
| |
IV |
trochlear |
m |
eye movement |
midbrain |
| |
V |
trigeminal |
m |
masticatory movements |
midbrain and pons |
| |
|
|
s |
sensitivity of face and tongue |
medulla |
| |
VI |
abducens |
m |
eye movements |
medulla |
| |
VII |
facial |
m |
facial movement |
medulla |
| |
VIII |
auditory |
s |
hearing |
medulla |
| |
|
vestibular |
s |
balance |
|
| |
IX |
glossopharyngeal |
s,m |
tongue and pharynx |
medulla |
| |
X |
vagus |
s,m |
heart, blood vessels, viscera |
medulla |
| |
XI |
spinal accessory |
m |
neck muscles and viscera |
medulla |
| |
XII |
hypoglossal |
m |
|
medulla |
|
REFERENCES
Fatt P, Katz B (1951): An analysis of the end-plate potential recorded with an intracellular electrode. J. Physiol. (Lond.) 115: 320-70.
Granit R, Haase J, Rutledge LT (1960): Recurrent inhibition in relation to frequency of firing and limitation of discharge rate of extensor motoneurons. J. Physiol. (Lond.) 154: 308-28.
Granit R, Renkin B (1961): Net depolarization and discharge rate of motoneurons, as measured by recurrent inhibition. J. Physiol. (Lond.) 158: 461-75.
Hille B (1970): Ionic channels in nerve membranes. Prog. Biophys. Mol. Biol. 21: 1-32.
Loewenstein WR (1959): The generation of electric activity in a nerve ending. Ann. N.Y. Acad. Sci. 81: 367-87.
Schmidt RF (ed.) (1981): Fundamentals of Sensory Physiology, 2nd ed., 286 pp. Springer-Verlag, New York, Heidelberg, Berlin.
Stevens CF (1968): Synaptic physiology. Proc. IEEE 56:(6) 916-30. (Special issue on studies of neural elements and systems).
Takeuchi A, Takeuchi N (1960): On the permeability of end-plate membrane during the action of transmitter. J. Physiol. (Lond.) 154: 52-67.
REFERENCES, BOOKS
Granit R (1955): Receptors and Sensory Perception, 369 pp. Yale University Press, New Haven.
Hille B (1992): Ionic Channels of Excitable Membranes, 2nd ed., 607 pp. Sinauer Assoc., Sunderland, Mass. (1st ed., 1984)
Jewett DL, Rayner MD (1984): Basic Concepts of Neuronal Function, 411 pp. Little Brown, Boston.
Kiloh LG, McComas AJ, Osselton JW (1981): Clinical Electroencephalography, 4th ed., 239 pp. Butterworth, London.
Kuffler SW, Nicholls JG, Martin AR (1984): From Neuron to Brain, 2nd ed., 651 pp. Sinauer Assoc., Sunderland, Mass.
Nunez PL (1981): Electric Fields of the Brain: The Neurophysics of EEG, 484 pp. Oxford University Press, New York.
Patton HD, Fuchs AF, Hille B, Scher AM, Steiner R (eds.) (1989): Textbook of Physiology, 21st ed., 1596 pp. W. B. Saunders, Philadelphia.
Penfield W, Rasmussen T (1950): The Cerebral Cortex of Man: A Clinical Study of Localization of Function, 248 pp. Macmillan, New York.
Schmidt RF (ed.) (1981): Fundamentals of Sensory Physiology, 2nd ed., 286 pp. Springer-Verlag, New York, Heidelberg, Berlin.
Shepherd GM (1988): Neurobiology, 689 pp. Oxford University Press, New York.
Williams PL, Warwick R (eds.) (1989): Gray's Anatomy, 37th ed., 1598 pp. Churchill Livingstone, Edinburgh.



 A discussion of the nervous system might logically begin with sensory cells located at the periphery of the body. These cells initiate and conduct signals to the brain and provide various sensory inputs such as vision, hearing, posture, and so on. Providing information on the environment to the body, these peripheral cells respond to stimuli with action pulses, which convey their information through encoded signals. These signals are conducted axonally through ascending pathways, across synapses, and finally to specific sites in the brain. Other neural cells in the brain process the coded signals, and direct the actions of muscles and other organs in response to the various sensory inputs. The entire circuit is recognized as a reflex arc, a basic unit in the nervous system. In some cases it is entirely automatic, and in others it is under voluntary control.
A discussion of the nervous system might logically begin with sensory cells located at the periphery of the body. These cells initiate and conduct signals to the brain and provide various sensory inputs such as vision, hearing, posture, and so on. Providing information on the environment to the body, these peripheral cells respond to stimuli with action pulses, which convey their information through encoded signals. These signals are conducted axonally through ascending pathways, across synapses, and finally to specific sites in the brain. Other neural cells in the brain process the coded signals, and direct the actions of muscles and other organs in response to the various sensory inputs. The entire circuit is recognized as a reflex arc, a basic unit in the nervous system. In some cases it is entirely automatic, and in others it is under voluntary control.
 No neurons run directly from the periphery to the brain. Normally the initiated signal is relayed by several intermediate neural cells. The interconnection between neurons, called the synapse, behaves as a simple switch but also has a special role in information processing. The junction (synapse) between a neural cell and the muscle that it innervates, called the neuromuscular junction, has been particularly well studied and provides much of our quantitative understanding about synapses. Since it is impossible to discuss the structure of the nervous system without including synapses, we begin our discussion with an examination of that topic.
No neurons run directly from the periphery to the brain. Normally the initiated signal is relayed by several intermediate neural cells. The interconnection between neurons, called the synapse, behaves as a simple switch but also has a special role in information processing. The junction (synapse) between a neural cell and the muscle that it innervates, called the neuromuscular junction, has been particularly well studied and provides much of our quantitative understanding about synapses. Since it is impossible to discuss the structure of the nervous system without including synapses, we begin our discussion with an examination of that topic.
 In cardiac muscle the intercellular space between abutting cells is spanned by gap junctions, which provide a low-resistance path for the local circuit currents and may be regarded as an electric (myo-myo) synapse. (The gap, however, is not called a synaptic cleft.) This type of junction is discussed in a later chapter.
In cardiac muscle the intercellular space between abutting cells is spanned by gap junctions, which provide a low-resistance path for the local circuit currents and may be regarded as an electric (myo-myo) synapse. (The gap, however, is not called a synaptic cleft.) This type of junction is discussed in a later chapter.
 The presynaptic nerve fiber endings are generally enlarged to form terminal buttons or synaptic knobs. Inside these knobs are the vesicles that contain the chemical transmitters. The arrival of the action pulse opens voltage-gated Ca2+ channels that permit an influx of calcium ions. These in turn trigger the release into the synaptic gap, by exocytosis, of a number of the "prepackaged" vesicles containing the neurotransmitter.
The presynaptic nerve fiber endings are generally enlarged to form terminal buttons or synaptic knobs. Inside these knobs are the vesicles that contain the chemical transmitters. The arrival of the action pulse opens voltage-gated Ca2+ channels that permit an influx of calcium ions. These in turn trigger the release into the synaptic gap, by exocytosis, of a number of the "prepackaged" vesicles containing the neurotransmitter.
 On average, each neuron divides into perhaps 1000 synaptic endings. On the other hand, a single spinal motor neuron may have an average of 10,000 synaptic inputs. Based on this data, it is not surprising that the ratio of synapse to neurons in the human forebrain is estimated to be around 4×104. In neuro-neuro synapses, the postjunctional site may be a dendrite or cell body, but the former predominates.
On average, each neuron divides into perhaps 1000 synaptic endings. On the other hand, a single spinal motor neuron may have an average of 10,000 synaptic inputs. Based on this data, it is not surprising that the ratio of synapse to neurons in the human forebrain is estimated to be around 4×104. In neuro-neuro synapses, the postjunctional site may be a dendrite or cell body, but the former predominates.

 Synaptic inhibition occurs at nerve-nerve (neuro-neuro) junctions when presynaptic activity releases a transmitter that hyperpolarizes the postsynaptic membrane (i.e., makes its membrane voltage more negative). In theory, hyperpolarization could result from elevation of either potassium or chloride permeability because the equilibrium potential of each is more negative than the normal resting potential (which is influenced in the positive direction by the presence of sodium). In actuality, however, inhibition is due to elevated chloride permeability.
Synaptic inhibition occurs at nerve-nerve (neuro-neuro) junctions when presynaptic activity releases a transmitter that hyperpolarizes the postsynaptic membrane (i.e., makes its membrane voltage more negative). In theory, hyperpolarization could result from elevation of either potassium or chloride permeability because the equilibrium potential of each is more negative than the normal resting potential (which is influenced in the positive direction by the presence of sodium). In actuality, however, inhibition is due to elevated chloride permeability.
 In contrast with the neuromuscular (neuro-myo) junction, a single excitatory input to a neuro-neuro synapse is completely inadequate to depolarize the postjunctional membrane to threshold. In fact, with perhaps thousands of both excitatory and inhibitory inputs on the postjunctional cell, a spatial and temporal summation is continually taking place, and the membrane voltage will fluctuate. When, finally, a threshold of perhaps 10-15 mV is reached, an action potential results. In this way, an important integrative process takes place at the inputs to each nerve cell. The reader with computer science experience can appreciate the tremendous possibilities for information processing that can (and do!) take place, particularly when one considers that there are perhaps 1012 neurons and 1015 synapses in the human brain. This is indeed a neural net.
In contrast with the neuromuscular (neuro-myo) junction, a single excitatory input to a neuro-neuro synapse is completely inadequate to depolarize the postjunctional membrane to threshold. In fact, with perhaps thousands of both excitatory and inhibitory inputs on the postjunctional cell, a spatial and temporal summation is continually taking place, and the membrane voltage will fluctuate. When, finally, a threshold of perhaps 10-15 mV is reached, an action potential results. In this way, an important integrative process takes place at the inputs to each nerve cell. The reader with computer science experience can appreciate the tremendous possibilities for information processing that can (and do!) take place, particularly when one considers that there are perhaps 1012 neurons and 1015 synapses in the human brain. This is indeed a neural net.
 Presynaptic inhibition is another inhibition mechanism. In this case an inhibitory nerve ending (from another axon known as the presynaptic inhibitor) is synapsed to an excitatory presynaptic terminal. The inhibitory nerve releases a transmitter that partially depolarizes the presynaptic cell. As a consequence, activation arising in the presynaptic fiber is diminished, hence the release of transmitter is reduced. As a result, the degree of excitation produced in the postsynaptic cell is reduced (hence an inhibitory effect).
Presynaptic inhibition is another inhibition mechanism. In this case an inhibitory nerve ending (from another axon known as the presynaptic inhibitor) is synapsed to an excitatory presynaptic terminal. The inhibitory nerve releases a transmitter that partially depolarizes the presynaptic cell. As a consequence, activation arising in the presynaptic fiber is diminished, hence the release of transmitter is reduced. As a result, the degree of excitation produced in the postsynaptic cell is reduced (hence an inhibitory effect).
 The falling phase of the EPSP is characterized by a single time constant - that is, the time required for the response to a single excitatory stimulus to diminish to 1/e of its maximum. This is an important value. If a sequence of afferent stimuli occurs in a very short time interval, then temporal summation of the EPSPs occurs, yielding a growing potential. Similarly, if activity occurs at more than one synaptic knob simultaneously (or within the length of the aforementioned time constant), then spatial summation results. The additive effect on a synapse is nonlinear. Furthermore, the individual synapses interact in an extremely complicated way (Stevens, 1968). Despite these complexities, it has been shown experimentally that both spatial and temporal summation generally behave in a simple linear manner (Granit, Haase, and Rutledge, 1960; Granit and Renkin, 1961).
The falling phase of the EPSP is characterized by a single time constant - that is, the time required for the response to a single excitatory stimulus to diminish to 1/e of its maximum. This is an important value. If a sequence of afferent stimuli occurs in a very short time interval, then temporal summation of the EPSPs occurs, yielding a growing potential. Similarly, if activity occurs at more than one synaptic knob simultaneously (or within the length of the aforementioned time constant), then spatial summation results. The additive effect on a synapse is nonlinear. Furthermore, the individual synapses interact in an extremely complicated way (Stevens, 1968). Despite these complexities, it has been shown experimentally that both spatial and temporal summation generally behave in a simple linear manner (Granit, Haase, and Rutledge, 1960; Granit and Renkin, 1961).
 Synaptic transmission has been compared to an electric information transfer circuit in the following way: In the nerve axon the information is transferred by means of nerve impulses in "digital" or, more accurately, "pulse-code modulated" form. In the synapse, information is conducted with the transmitter substance in analog form, to be converted again in the next neuron into "digital" form. Though this analogy is not correct in all aspects, it illustrates the character of the neural information chain.
Synaptic transmission has been compared to an electric information transfer circuit in the following way: In the nerve axon the information is transferred by means of nerve impulses in "digital" or, more accurately, "pulse-code modulated" form. In the synapse, information is conducted with the transmitter substance in analog form, to be converted again in the next neuron into "digital" form. Though this analogy is not correct in all aspects, it illustrates the character of the neural information chain.
 The reflex arc, illustrated above, is considered to be the basic unit of integrated neural activity. It consists essentially of a sensory receptor, an afferent neuron, one or more synapses, an efferent neuron, and a muscle or other effector. The connection between afferent and efferent pathways is found, generally, in the spinal cord or the brain. The simplest reflex involves only a single synapse between afferent and efferent neurons (a monosynaptic reflex); an example is the familiar knee jerk reflex.
The reflex arc, illustrated above, is considered to be the basic unit of integrated neural activity. It consists essentially of a sensory receptor, an afferent neuron, one or more synapses, an efferent neuron, and a muscle or other effector. The connection between afferent and efferent pathways is found, generally, in the spinal cord or the brain. The simplest reflex involves only a single synapse between afferent and efferent neurons (a monosynaptic reflex); an example is the familiar knee jerk reflex.
 Homeostasis refers to the various regulatory processes in the body that maintain a normal state in the face of disturbances. The autonomic nervous system is organized to accomplish this automatically with regard to many organs of the body; its activity, like that of the somatic nervous system, is based on the reflex arc. In this case signals, which arise at visceral receptors, are conveyed via afferent neurons to the central nervous system, where integration takes place, resulting in efferent signals to visceral effectors (in particular, smooth muscle) to restore or maintain normal conditions. Integration of signals affecting blood pressure and respiration takes place in the medulla oblongata; those controlling pupillary response to light are integrated in the midbrain, whereas those responding to body temperature are integrated in the hypothalamus - to give only a few examples.
Homeostasis refers to the various regulatory processes in the body that maintain a normal state in the face of disturbances. The autonomic nervous system is organized to accomplish this automatically with regard to many organs of the body; its activity, like that of the somatic nervous system, is based on the reflex arc. In this case signals, which arise at visceral receptors, are conveyed via afferent neurons to the central nervous system, where integration takes place, resulting in efferent signals to visceral effectors (in particular, smooth muscle) to restore or maintain normal conditions. Integration of signals affecting blood pressure and respiration takes place in the medulla oblongata; those controlling pupillary response to light are integrated in the midbrain, whereas those responding to body temperature are integrated in the hypothalamus - to give only a few examples.
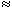 0). What happens if the membrane becomes equally permeable to sodium and potassium ions? Such a condition would alter the membrane potential from near the potassium Nernst potential to a value that approximates the average of the sodium and potassium equilibrium potentials. (This potential, in turn, is close to zero transmembrane voltage and is entirely adequate to initiate an activation.) If the postsynaptic region is voltage-clamped, the value that reduces the membrane current to zero during transmitter release is called the reversal voltage Vr. One can show that it equals the average Nernst potential of sodium and potassium, as mentioned above. In the neuromuscular junction in skeletal muscle, this reversal voltage is about -15 mV.
0). What happens if the membrane becomes equally permeable to sodium and potassium ions? Such a condition would alter the membrane potential from near the potassium Nernst potential to a value that approximates the average of the sodium and potassium equilibrium potentials. (This potential, in turn, is close to zero transmembrane voltage and is entirely adequate to initiate an activation.) If the postsynaptic region is voltage-clamped, the value that reduces the membrane current to zero during transmitter release is called the reversal voltage Vr. One can show that it equals the average Nernst potential of sodium and potassium, as mentioned above. In the neuromuscular junction in skeletal muscle, this reversal voltage is about -15 mV.
 The electric behavior at a synapse can be estimated by examining an equivalent circuit of the postsynaptic membrane, such as that shown in Figure 5.2. Two regions are identified: One represents the membrane associated with receptors sensitive to the transmitter, and the other the normal excitable membrane of the cell. In Figure 5.2 these two regions are represented by discrete elements, but in reality these are distributed along the structure that constitutes the actual cell. This figure depicts a neuromuscular junction, where the release of acetylcholine results in the elevation of sodium and potassium conductance in the target region, which is in turn depicted by the closing of the ACh switch. Upon closure of this switch,
The electric behavior at a synapse can be estimated by examining an equivalent circuit of the postsynaptic membrane, such as that shown in Figure 5.2. Two regions are identified: One represents the membrane associated with receptors sensitive to the transmitter, and the other the normal excitable membrane of the cell. In Figure 5.2 these two regions are represented by discrete elements, but in reality these are distributed along the structure that constitutes the actual cell. This figure depicts a neuromuscular junction, where the release of acetylcholine results in the elevation of sodium and potassium conductance in the target region, which is in turn depicted by the closing of the ACh switch. Upon closure of this switch,
 DINa = DGNa(Vm - VNa)
DINa = DGNa(Vm - VNa) DIK = DGK(Vm - VK)
DIK = DGK(Vm - VK)
 DINa = DGNa(VR - VNa)
DINa = DGNa(VR - VNa) DIK = DGK(VR - VK)
DIK = DGK(VR - VK) For the conditions described by Equations 5.3 and 5.4, since the total current at the reversal voltage is zero, it follows that the sodium and potassium ion currents are equal and opposite in sign (i.e., DINa = -DIK). Consequently, applying this condition to Equations 5.3 and 5.4 results in the following:
For the conditions described by Equations 5.3 and 5.4, since the total current at the reversal voltage is zero, it follows that the sodium and potassium ion currents are equal and opposite in sign (i.e., DINa = -DIK). Consequently, applying this condition to Equations 5.3 and 5.4 results in the following:
 DGNa(VR - VNa) = - DGK(VR - VK)
DGNa(VR - VNa) = - DGK(VR - VK) (DGNa + DGK) VR = DGNaVNa - DGKVK
(DGNa + DGK) VR = DGNaVNa - DGKVK
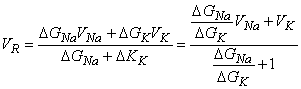
 From Equation 5.7 it is easy to see that if the introduction of ACh causes an equal increase in the sodium and potassium conductances - that is, if
From Equation 5.7 it is easy to see that if the introduction of ACh causes an equal increase in the sodium and potassium conductances - that is, if

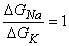

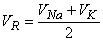
 Returning to Figure 5.2, and applying Thevenin's theorem, we can simplify the receptor circuit to consist of a single battery whose emf is the average of VNa and VK (hence VR), and with a conductivity gR = gNa + gK. Its effect on the normal membrane of the postsynaptic cell can be calculated since the total current at any node is necessarily zero - that is, there are no applied currents. Consequently,
Returning to Figure 5.2, and applying Thevenin's theorem, we can simplify the receptor circuit to consist of a single battery whose emf is the average of VNa and VK (hence VR), and with a conductivity gR = gNa + gK. Its effect on the normal membrane of the postsynaptic cell can be calculated since the total current at any node is necessarily zero - that is, there are no applied currents. Consequently,
 GR (Vm - VR) + GK(Vm - VK) + GNa(Vm - VNa) = 0
GR (Vm - VR) + GK(Vm - VK) + GNa(Vm - VNa) = 0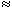 0 , as noted above. Solving for the postsynaptic potential Vm results in
0 , as noted above. Solving for the postsynaptic potential Vm results in

 There are at least a dozen conscious sense modalities with which we are familiar. In addition, there are other sensory receptors whose information processing goes on without our awareness. Together these may be classified as (1) extroreceptors, which sense stimuli arising external to the body; (2) introreceptors, which respond to physical or chemical qualities within the body; and (3) proprioceptors, which provide information on the body's position. Examples in each of these categories include the following:
There are at least a dozen conscious sense modalities with which we are familiar. In addition, there are other sensory receptors whose information processing goes on without our awareness. Together these may be classified as (1) extroreceptors, which sense stimuli arising external to the body; (2) introreceptors, which respond to physical or chemical qualities within the body; and (3) proprioceptors, which provide information on the body's position. Examples in each of these categories include the following:
 The sensory receptor contains membrane regions that respond to one of the various forms of incident stimuli by a depolarization (or hyperpolarization). In some cases the receptor is actually part of the afferent neuron but, in others it consists of a separate specialized cell. All receptor cells have a common feature: They are transducers - that is, they change energy from one form to another. For instance, the sense of touch in the skin arises from the conversion of mechanical and/or thermal energy into the electric energy (ionic currents) of the nerve impulse. In general, the receptor cells do not generate an activation impulse themselves. Instead, they generate a gradually increasing potential, which triggers activation of the afferent nerve fiber to which they are connected.
The sensory receptor contains membrane regions that respond to one of the various forms of incident stimuli by a depolarization (or hyperpolarization). In some cases the receptor is actually part of the afferent neuron but, in others it consists of a separate specialized cell. All receptor cells have a common feature: They are transducers - that is, they change energy from one form to another. For instance, the sense of touch in the skin arises from the conversion of mechanical and/or thermal energy into the electric energy (ionic currents) of the nerve impulse. In general, the receptor cells do not generate an activation impulse themselves. Instead, they generate a gradually increasing potential, which triggers activation of the afferent nerve fiber to which they are connected.
 The electric events in receptors may be separated into two distinct components:
The electric events in receptors may be separated into two distinct components:
 These voltage changes are, however, one and the same in a receptor such as the Pacinian corpuscle, in which there are no specialized receptor cells. But in cases like the retina where specialized receptor cells (i.e., the rods and cones) do exist, these voltages are separate. In the following, we consider the Pacinian corpuscle in more detail (Granit, 1955).
These voltage changes are, however, one and the same in a receptor such as the Pacinian corpuscle, in which there are no specialized receptor cells. But in cases like the retina where specialized receptor cells (i.e., the rods and cones) do exist, these voltages are separate. In the following, we consider the Pacinian corpuscle in more detail (Granit, 1955).
 Because the neural output is carried in the form of all-or-nothing action pulses, we must look to another form of signal than one that is amplitude modulated. In fact, the generator or receptor potentials cause repetitive firing of action pulses on the afferent neuron, and the firing rate (and rate of change) is reflective of the sensory input. This coded signal can be characteristic of the modality being transduced.
Because the neural output is carried in the form of all-or-nothing action pulses, we must look to another form of signal than one that is amplitude modulated. In fact, the generator or receptor potentials cause repetitive firing of action pulses on the afferent neuron, and the firing rate (and rate of change) is reflective of the sensory input. This coded signal can be characteristic of the modality being transduced.
 In a process of adaptation, the frequency of action potential firing decreases in time with respect to a steady stimulus. One can separate the responses into fast and slow rates of adaptation, depending on how quickly the frequency reduction takes place (i.e., muscle spindle is slow whereas touch is fast).
In a process of adaptation, the frequency of action potential firing decreases in time with respect to a steady stimulus. One can separate the responses into fast and slow rates of adaptation, depending on how quickly the frequency reduction takes place (i.e., muscle spindle is slow whereas touch is fast).

 Werner R. Loewenstein (1959) stimulated the corpuscle with a piezoelectric crystal and measured the generator voltage (from the unmyelinated terminal axon) and the action potential (from the nodes of Ranvier) with an external electrode. He peeled off the layers of the corpuscle, and even after the last layer was removed, the corpuscle generated signals similar to those observed with the capsule intact (see recordings shown in Figure 5.4).
Werner R. Loewenstein (1959) stimulated the corpuscle with a piezoelectric crystal and measured the generator voltage (from the unmyelinated terminal axon) and the action potential (from the nodes of Ranvier) with an external electrode. He peeled off the layers of the corpuscle, and even after the last layer was removed, the corpuscle generated signals similar to those observed with the capsule intact (see recordings shown in Figure 5.4).

 (A) The normal response of the generator voltage for increasing applied force (a)-(e).
(A) The normal response of the generator voltage for increasing applied force (a)-(e).
 (B) The layers of the corpuscle have been removed, leaving the nerve terminal intact. The response to application of mechanical force is unchanged from A.
(B) The layers of the corpuscle have been removed, leaving the nerve terminal intact. The response to application of mechanical force is unchanged from A.
 (C) Partial destruction of the core sheath does not change the response from A or B.
(C) Partial destruction of the core sheath does not change the response from A or B.
 (D) Blocking the first node of Ranvier eliminates the initiation of the activation process but does not interfere with the formation of the generator voltage.
(D) Blocking the first node of Ranvier eliminates the initiation of the activation process but does not interfere with the formation of the generator voltage.
 (E) Degeneration of the nerve ending prevents the creation of the generator voltage.
(E) Degeneration of the nerve ending prevents the creation of the generator voltage. The generator voltage has properties similar to these of the excitatory postsynaptic voltage. (The generator voltage is a graded response whereby a weak stimulus generates a low generator voltage whereas a strong stimulus generates a large generator voltage.) Even partial destruction of the corpuscle did not prevent it from producing a generator voltage. But when Loewenstein destroyed the nerve ending itself, a generator voltage could no longer be elicited. This observation formed the basis for supposing that the transducer itself was located in the nerve ending. The generator voltage does not propagate on the nerve fiber (in fact, the nerve ending is electrically inexcitable) but, rather, triggers the activation process in the first node of Ranvier by electrotonic (passive) conduction. If the first node is blocked, no activation is initiated in the nerve fiber.
The generator voltage has properties similar to these of the excitatory postsynaptic voltage. (The generator voltage is a graded response whereby a weak stimulus generates a low generator voltage whereas a strong stimulus generates a large generator voltage.) Even partial destruction of the corpuscle did not prevent it from producing a generator voltage. But when Loewenstein destroyed the nerve ending itself, a generator voltage could no longer be elicited. This observation formed the basis for supposing that the transducer itself was located in the nerve ending. The generator voltage does not propagate on the nerve fiber (in fact, the nerve ending is electrically inexcitable) but, rather, triggers the activation process in the first node of Ranvier by electrotonic (passive) conduction. If the first node is blocked, no activation is initiated in the nerve fiber.
 The ionic flow mechanism underlying the generator (receptor) voltage is the same as that for the excitatory postsynaptic voltage. Thus deformation of the Pacinian corpuscle increases both the sodium and potassium conductances such that their ratio (PNa/PK) increases and depolarization of the membrane potential results. As a result, the following behavior is observed:
The ionic flow mechanism underlying the generator (receptor) voltage is the same as that for the excitatory postsynaptic voltage. Thus deformation of the Pacinian corpuscle increases both the sodium and potassium conductances such that their ratio (PNa/PK) increases and depolarization of the membrane potential results. As a result, the following behavior is observed:

 The entire human brain weighs about 1500 g (Williams and Warwick, 1989). In the brain the cerebrum is the largest part. The surface of the cerebrum is strongly folded. These folds are divided into two hemispheres which are separated by a deep fissure and connected by the corpus callosum. Existing within the brain are three ventricles containing cerebrospinal fluid. The hemispheres are divided into the following lobes: lobus frontalis, lobus parietalis, lobus occipitalis, and lobus temporalis. The surface area of the cerebrum is about 1600 cm², and its thickness is 3 mm. Six layers, or laminae, each consisting of different neuronal types and populations, can be observed in this surface layer. The higher cerebral functions, accurate sensations, and the voluntary motor control of muscles are located in this region.
The entire human brain weighs about 1500 g (Williams and Warwick, 1989). In the brain the cerebrum is the largest part. The surface of the cerebrum is strongly folded. These folds are divided into two hemispheres which are separated by a deep fissure and connected by the corpus callosum. Existing within the brain are three ventricles containing cerebrospinal fluid. The hemispheres are divided into the following lobes: lobus frontalis, lobus parietalis, lobus occipitalis, and lobus temporalis. The surface area of the cerebrum is about 1600 cm², and its thickness is 3 mm. Six layers, or laminae, each consisting of different neuronal types and populations, can be observed in this surface layer. The higher cerebral functions, accurate sensations, and the voluntary motor control of muscles are located in this region.
 The interbrain or diencephalon is surrounded by the cerebrum and is located around the third ventricle. It includes the thalamus, which is a bridge connecting the sensory paths. The hypothalamus, which is located in the lower part of the interbrain, is important for the regulation of autonomic (involuntary) functions. Together with the hypophysis, it regulates hormonal secretions. The midbrain is a small part of the brain. The pons Varolii is an interconnection of neural tracts; the cerebellum controls fine movement. The medulla oblongata resembles the spinal cord to which it is immediately connected. Many reflex centers, such as the vasomotor center and the breathing center, are located in the medulla oblongata.
The interbrain or diencephalon is surrounded by the cerebrum and is located around the third ventricle. It includes the thalamus, which is a bridge connecting the sensory paths. The hypothalamus, which is located in the lower part of the interbrain, is important for the regulation of autonomic (involuntary) functions. Together with the hypophysis, it regulates hormonal secretions. The midbrain is a small part of the brain. The pons Varolii is an interconnection of neural tracts; the cerebellum controls fine movement. The medulla oblongata resembles the spinal cord to which it is immediately connected. Many reflex centers, such as the vasomotor center and the breathing center, are located in the medulla oblongata.
 In the cerebral cortex one may locate many different areas of specialized brain function (Penfield and Rasmussen, 1950; Kiloh, McComas, and Osselton, 1981). The higher brain functions occur in the frontal lobe, the visual center is located in the occipital lobe, and the sensory area and motor area are located on both sides of the central fissure. There are specific areas in the sensory and motor cortex whose elements correspond to certain parts of the body. The size of each such area is proportional to the required accuracy of sensory or motor control. These regions are described in Figure 5.6. Typically, the sensory areas represented by the lips and the hands are large, and the areas represented by the midbody and eyes are small. The visual center is located in a different part of the brain. The motor area, the area represented by the hands and the speaking organs, is large.
In the cerebral cortex one may locate many different areas of specialized brain function (Penfield and Rasmussen, 1950; Kiloh, McComas, and Osselton, 1981). The higher brain functions occur in the frontal lobe, the visual center is located in the occipital lobe, and the sensory area and motor area are located on both sides of the central fissure. There are specific areas in the sensory and motor cortex whose elements correspond to certain parts of the body. The size of each such area is proportional to the required accuracy of sensory or motor control. These regions are described in Figure 5.6. Typically, the sensory areas represented by the lips and the hands are large, and the areas represented by the midbody and eyes are small. The visual center is located in a different part of the brain. The motor area, the area represented by the hands and the speaking organs, is large.

 In the area of vision, afferent fibers from the photoreceptors carry signals to the brain stem through the optic nerve and optic tract to synapse in the lateral geniculate body (a part of the thalamus). From here axons pass to the occipital lobe of the cerebral cortex. In addition, branches of the axons of the optic tract synapse with neurons in the zone between thalamus and midbrain which is the pretectal nucleus and superior colliculus. These, in turn, synapse with preganglionic parasympathetic neurons whose axons follow the oculomotor nerve to the ciliary ganglion (located just behind the eyeball). The reflex loop is closed by postganglionic fibers which pass along ciliary nerves to the iris muscles (controlling pupil aperture) and to muscles controlling the lens curvature (adjusting its refractive or focusing qualities). Other reflexes concerned with head and/or eye movements may also be initiated.
In the area of vision, afferent fibers from the photoreceptors carry signals to the brain stem through the optic nerve and optic tract to synapse in the lateral geniculate body (a part of the thalamus). From here axons pass to the occipital lobe of the cerebral cortex. In addition, branches of the axons of the optic tract synapse with neurons in the zone between thalamus and midbrain which is the pretectal nucleus and superior colliculus. These, in turn, synapse with preganglionic parasympathetic neurons whose axons follow the oculomotor nerve to the ciliary ganglion (located just behind the eyeball). The reflex loop is closed by postganglionic fibers which pass along ciliary nerves to the iris muscles (controlling pupil aperture) and to muscles controlling the lens curvature (adjusting its refractive or focusing qualities). Other reflexes concerned with head and/or eye movements may also be initiated.
 Motor signals to muscles of the trunk and periphery from higher motor centers of the cerebral cortex first travel along upper motor neurons to the medulla oblongata. From here most of the axons of the upper motor neurons cross to the other side of the central nervous system and descend the spinal cord in the lateral corticospinal tract; the remainder travel down the cord in the anterior corticospinal tract. The upper motor neurons eventually synapse with lower motor neurons in the ventral horn of the spinal cord; the lower motor neurons complete the path to the target muscles. Most reflex motor movements involve complex neural integration and coordinate signals to the muscles involved in order to achieve a smooth performance.
Motor signals to muscles of the trunk and periphery from higher motor centers of the cerebral cortex first travel along upper motor neurons to the medulla oblongata. From here most of the axons of the upper motor neurons cross to the other side of the central nervous system and descend the spinal cord in the lateral corticospinal tract; the remainder travel down the cord in the anterior corticospinal tract. The upper motor neurons eventually synapse with lower motor neurons in the ventral horn of the spinal cord; the lower motor neurons complete the path to the target muscles. Most reflex motor movements involve complex neural integration and coordinate signals to the muscles involved in order to achieve a smooth performance.
 Effective integration of sensory information requires that this information be collected at a single center. In the cerebral cortex, one can indeed locate specific areas identified with specific sensory inputs (Penfield and Rasmussen, 1950; Kiloh, McComas, and Osselton, 1981). While the afferent signals convey information regarding stimulus strength, recognition of the modality depends on pinpointing the anatomical classification of the afferent pathways. (This can be demonstrated by interchanging the afferent fibers from, say, auditory and tactile receptors, in which case sound inputs are perceived as of tactile origin and vice versa.)
Effective integration of sensory information requires that this information be collected at a single center. In the cerebral cortex, one can indeed locate specific areas identified with specific sensory inputs (Penfield and Rasmussen, 1950; Kiloh, McComas, and Osselton, 1981). While the afferent signals convey information regarding stimulus strength, recognition of the modality depends on pinpointing the anatomical classification of the afferent pathways. (This can be demonstrated by interchanging the afferent fibers from, say, auditory and tactile receptors, in which case sound inputs are perceived as of tactile origin and vice versa.)
 The higher brain functions take place in the frontal lobe, the visual center is in the occipital lobe, the sensory area and motor area are located on both sides of the central fissure. As described above, there is an area in the sensory cortex whose elements correspond to each part of the body. In a similar way, a part of the brain contains centers for generating command (efferent) signals for control of the body's musculature. Here, too, one finds projections from specific cortical areas to specific parts of the body.
The higher brain functions take place in the frontal lobe, the visual center is in the occipital lobe, the sensory area and motor area are located on both sides of the central fissure. As described above, there is an area in the sensory cortex whose elements correspond to each part of the body. In a similar way, a part of the brain contains centers for generating command (efferent) signals for control of the body's musculature. Here, too, one finds projections from specific cortical areas to specific parts of the body.


