6.1 ANATOMY AND PHYSIOLOGY OF THE HEART
6.1.1 Location of the Heart
The heart is located in the chest between the lungs behind the sternum and above the diaphragm. It is surrounded by the pericardium. Its size is about that of a fist, and its weight is about 250-300 g. Its center is located about 1.5 cm to the left of the midsagittal plane. Located above the heart are the great vessels: the superior and inferior vena cava, the pulmonary artery and vein, as well as the aorta. The aortic arch lies behind the heart. The esophagus and the spine lie further behind the heart. An overall view is given in Figure 6.1 (Williams and Warwick, 1989)..
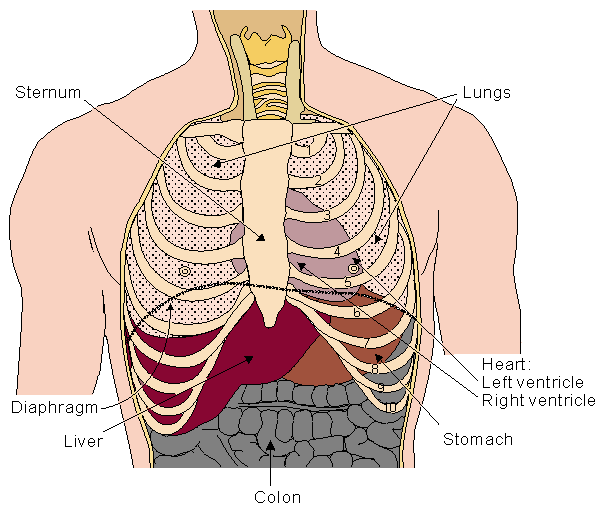
Fig. 6.1. Location of the heart in the thorax. It is bounded by the diaphragm, lungs, esophagus, descending aorta, and sternum.
6.1.2 Anatomy of the Heart
The walls of the heart are composed of cardiac muscle, called myocardium. It also has striations similar to skeletal muscle. It consists of four compartments: the right and left atria and ventricles. The heart is oriented so that the anterior aspect is the right ventricle while the posterior aspect shows the left atrium (see Figure 6.2). The atria form one unit and the ventricles another. This has special importance to the electric function of the heart, which will be discussed later. The left ventricular free wall and the septum are much thicker than the right ventricular wall. This is logical since the left ventricle pumps blood to the systemic circulation, where the pressure is considerably higher than for the pulmonary circulation, which arises from right ventricular outflow. The cardiac muscle fibers are oriented spirally (see Figure 6.3) and are divided into four groups: Two groups of fibers wind around the outside of both ventricles. Beneath these fibers a third group winds around both ventricles. Beneath these fibers a fourth group winds only around the left ventricle. The fact that cardiac muscle cells are oriented more tangentially than radially, and that the resistivity of the muscle is lower in the direction of the fiber has importance in electrocardiography and magnetocardiography.
The cardiac muscle fibers are oriented spirally (see Figure 6.3) and are divided into four groups: Two groups of fibers wind around the outside of both ventricles. Beneath these fibers a third group winds around both ventricles. Beneath these fibers a fourth group winds only around the left ventricle. The fact that cardiac muscle cells are oriented more tangentially than radially, and that the resistivity of the muscle is lower in the direction of the fiber has importance in electrocardiography and magnetocardiography.
 The heart has four valves. Between the right atrium and ventricle lies the tricuspid valve, and between the left atrium and ventricle is the mitral valve. The pulmonary valve lies between the right ventricle and the pulmonary artery, while the aortic valve lies in the outflow tract of the left ventricle (controlling flow to the aorta).
The heart has four valves. Between the right atrium and ventricle lies the tricuspid valve, and between the left atrium and ventricle is the mitral valve. The pulmonary valve lies between the right ventricle and the pulmonary artery, while the aortic valve lies in the outflow tract of the left ventricle (controlling flow to the aorta).
 The blood returns from the systemic circulation to the right atrium and from there goes through the tricuspid valve to the right ventricle. It is ejected from the right ventricle through the pulmonary valve to the lungs. Oxygenated blood returns from the lungs to the left atrium, and from there through the mitral valve to the left ventricle. Finally blood is pumped through the aortic valve to the aorta and the systemic circulation..
The blood returns from the systemic circulation to the right atrium and from there goes through the tricuspid valve to the right ventricle. It is ejected from the right ventricle through the pulmonary valve to the lungs. Oxygenated blood returns from the lungs to the left atrium, and from there through the mitral valve to the left ventricle. Finally blood is pumped through the aortic valve to the aorta and the systemic circulation..
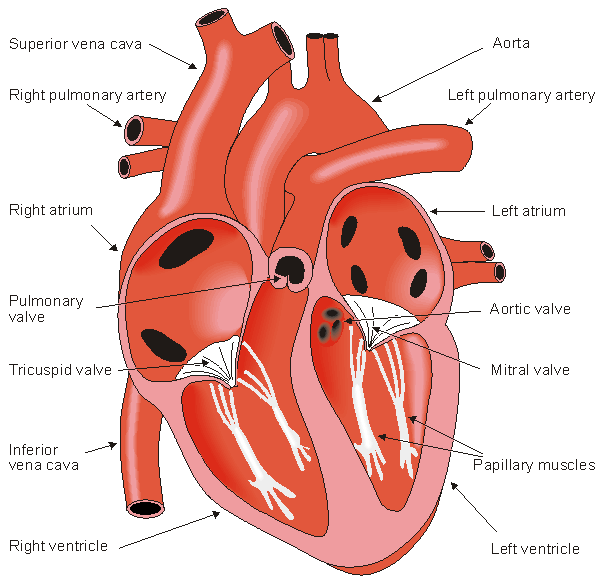
- Fig. 6.2. The anatomy of the heart and associated vessels.
Because the intrinsic rate of the sinus node is the greatest, it sets the activation frequency of the whole heart. If the connection from the atria to the AV node fails, the AV node adopts its intrinsic frequency. If the conduction system fails at the bundle of His, the ventricles will beat at the rate determined by their own region that has the highest intrinsic frequency. The electric events in the heart are summarized in Table 6.1. The waveforms of action impulse observed in different specialized cardiac tissue are shown in Figure 6.7.
bundle of His
epicardium
epicardium
endocardium
depolarization
depolarization
repolarization
175
400
1.0-1.5
0.3 (axial)
Fig. 6.7. Electrophysiology of the heart.The different waveforms for each of the specialized cells found in the heart are shown. The latency shown approximates that normally found in the healthy heart.
A classical study of the propagation of excitation in human heart was made by Durrer and his co-workers (Durrer et al., 1970). They isolated the heart from a subject who had died of various cerebral conditions and who had no previous history of cardiac diseases. The heart was removed within 30 min post mortem and was perfused. As many as 870 electrodes were placed into the cardiac muscle; the electric activity was then recorded by a tape recorder and played back at a lower speed by the ECG writer; thus the effective paper speed was 960 mm/s, giving a time resolution better than 1 ms.
Figure 6.8 is redrawn from these experimental data. The ventricles are shown with the anterior wall of the left and partly that of the right ventricle opened. The isochronic surfaces show clearly that ventricular activation starts from the inner wall of the left ventricle and proceeds radially toward the epicardium. In the terminal part of ventricular activation, the excitation wavefront proceeds more tangentially. This phenomenon and its effects on electrocardiogram and magnetocardiogram signals are discussed in greater detail later.
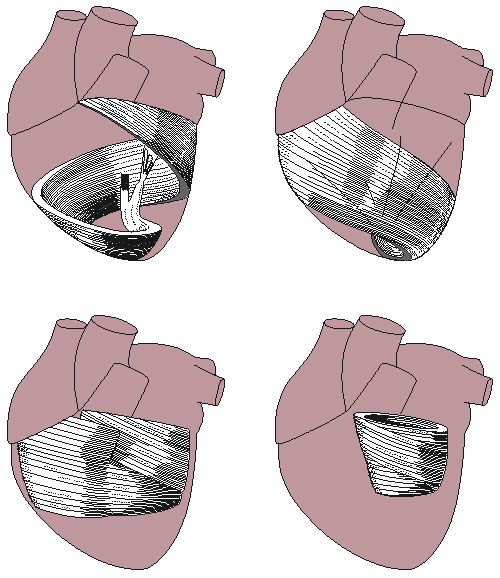
Fig. 6.3. Orientation of cardiac muscle fibers.
6.2 ELECTRIC ACTIVATION OF THE HEART
6.2.1 Cardiac Muscle Cell
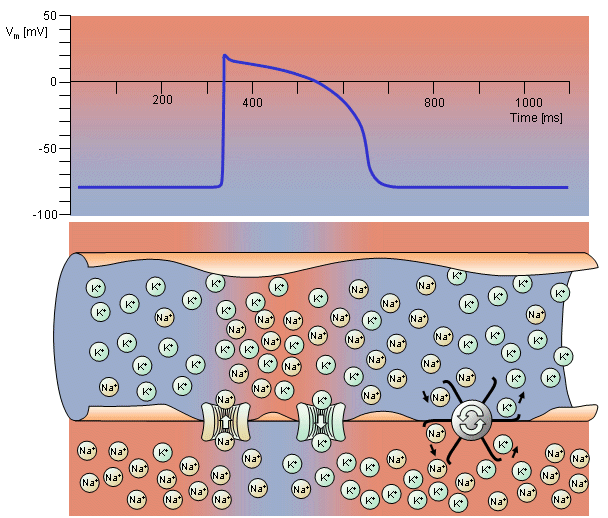
In the heart muscle cell, or myocyte, electric activation takes place by means of the same mechanism as in the nerve cell - that is, from the inflow of sodium ions across the cell membrane. The amplitude of the action potential is also similar, being about 100 mV for both nerve and muscle. The duration of the cardiac muscle impulse is, however, two orders of magnitude longer than that in either nerve cell or skeletal muscle. A plateau phase follows cardiac depolarization, and thereafter repolarization takes place. As in the nerve cell, repolarization is a consequence of the outflow of potassium ions. The duration of the action impulse is about 300 ms, as shown in Figure 6.4 (Netter, 1971).
 Associated with the electric activation of cardiac muscle cell is its mechanical contraction, which occurs a little later. For the sake of comparison, Figure 6.5 illustrates the electric activity and mechanical contraction of frog sartorius muscle, frog cardiac muscle, and smooth muscle from the rat uterus (Ruch and Patton, 1982).
Associated with the electric activation of cardiac muscle cell is its mechanical contraction, which occurs a little later. For the sake of comparison, Figure 6.5 illustrates the electric activity and mechanical contraction of frog sartorius muscle, frog cardiac muscle, and smooth muscle from the rat uterus (Ruch and Patton, 1982).
 An important distinction between cardiac muscle tissue and skeletal muscle is that in cardiac muscle, activation can propagate from one cell to another in any direction. As a result, the activation wavefronts are of rather complex shape. The only exception is the boundary between the atria and ventricles, which the activation wave normally cannot cross except along a special conduction system, since a nonconducting barrier of fibrous tissue is present..
An important distinction between cardiac muscle tissue and skeletal muscle is that in cardiac muscle, activation can propagate from one cell to another in any direction. As a result, the activation wavefronts are of rather complex shape. The only exception is the boundary between the atria and ventricles, which the activation wave normally cannot cross except along a special conduction system, since a nonconducting barrier of fibrous tissue is present..
DEPOLARIZATION REPOLARIZATION RESTORATION OF IONIC BALANCE
Fig. 6.4. Electrophysiology of the cardiac muscle cell.
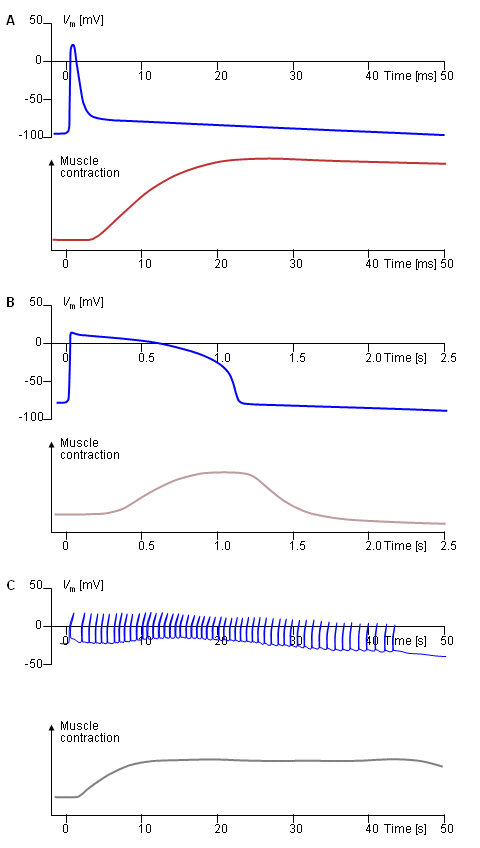
Fig. 6.5. Electric and mechanical activity in
 (A) frog sartorius muscle cell,
(A) frog sartorius muscle cell,
 (B) frog cardiac muscle cell, and
(B) frog cardiac muscle cell, and
 (C) rat uterus wall smooth muscle cell.
(C) rat uterus wall smooth muscle cell.
In each section the upper curve shows the transmembrane voltage behavior, whereas the lower one describes the mechanical contraction associated with it.6.2.2 The Conduction System of the Heart
Located in the right atrium at the superior vena cava is the sinus node (sinoatrial or SA node) which consists of specialized muscle cells. The sinoatrial node in humans is in the shape of a crescent and is about 15 mm long and 5 mm wide (see Figure 6.6). The SA nodal cells are self-excitatory, pacemaker cells. They generate an action potential at the rate of about 70 per minute. From the sinus node, activation propagates throughout the atria, but cannot propagate directly across the boundary between atria and ventricles, as noted above.
 The atrioventricular node (AV node) is located at the boundary between the atria and ventricles; it has an intrinsic frequency of about 50 pulses/min. However, if the AV node is triggered with a higher pulse frequency, it follows this higher frequency. In a normal heart, the AV node provides the only conducting path from the atria to the ventricles. Thus, under normal conditions, the latter can be excited only by pulses that propagate through it.
The atrioventricular node (AV node) is located at the boundary between the atria and ventricles; it has an intrinsic frequency of about 50 pulses/min. However, if the AV node is triggered with a higher pulse frequency, it follows this higher frequency. In a normal heart, the AV node provides the only conducting path from the atria to the ventricles. Thus, under normal conditions, the latter can be excited only by pulses that propagate through it.
 Propagation from the AV node to the ventricles is provided by a specialized conduction system. Proximally, this system is composed of a common bundle, called the bundle of His (named after German physician Wilhelm His, Jr., 1863-1934). More distally, it separates into two bundle branches propagating along each side of the septum, constituting the right and left bundle branches. (The left bundle subsequently divides into an anterior and posterior branch.) Even more distally the bundles ramify into Purkinje fibers (named after Jan Evangelista Purkinje (Czech; 1787-1869)) that diverge to the inner sides of the ventricular walls. Propagation along the conduction system takes place at a relatively high speed once it is within the ventricular region, but prior to this (through the AV node) the velocity is extremely slow.
Propagation from the AV node to the ventricles is provided by a specialized conduction system. Proximally, this system is composed of a common bundle, called the bundle of His (named after German physician Wilhelm His, Jr., 1863-1934). More distally, it separates into two bundle branches propagating along each side of the septum, constituting the right and left bundle branches. (The left bundle subsequently divides into an anterior and posterior branch.) Even more distally the bundles ramify into Purkinje fibers (named after Jan Evangelista Purkinje (Czech; 1787-1869)) that diverge to the inner sides of the ventricular walls. Propagation along the conduction system takes place at a relatively high speed once it is within the ventricular region, but prior to this (through the AV node) the velocity is extremely slow.
 From the inner side of the ventricular wall, the many activation sites cause the formation of a wavefront which propagates through the ventricular mass toward the outer wall. This process results from cell-to-cell activation. After each ventricular muscle region has depolarized, repolarization occurs. Repolarization is not a propagating phenomenon, and because the duration of the action impulse is much shorter at the epicardium (the outer side of the cardiac muscle) than at the endocardium (the inner side of the cardiac muscle), the termination of activity appears as if it were propagating from epicardium toward the endocardium.
From the inner side of the ventricular wall, the many activation sites cause the formation of a wavefront which propagates through the ventricular mass toward the outer wall. This process results from cell-to-cell activation. After each ventricular muscle region has depolarized, repolarization occurs. Repolarization is not a propagating phenomenon, and because the duration of the action impulse is much shorter at the epicardium (the outer side of the cardiac muscle) than at the endocardium (the inner side of the cardiac muscle), the termination of activity appears as if it were propagating from epicardium toward the endocardium.
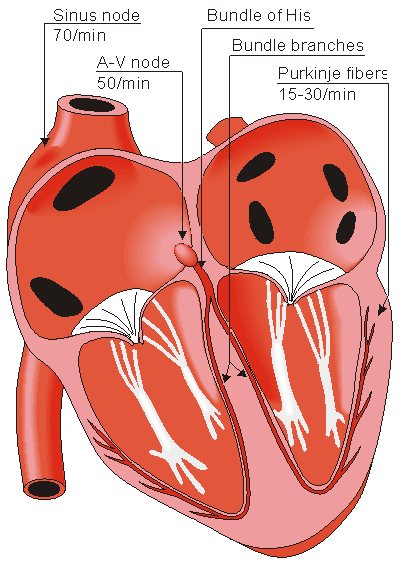
Fig. 6.6. The conduction system of the heart.
Location in
the heartEvent Time [ms]
ECG-
terminologyConduction
velocity [m/s] Intrinsic
frequency [1/min]
SA node
atrium, Right
Left
AV node
bundle branches
Purkinje fibers
endocardium
Septum
Left ventricle
Left ventricle
Right ventricle
Left ventricle
Right ventricle
Left ventricle
impulse generated
depolarization *)
depolarization
arrival of impulse
departure of impulse
activated
activated
activated
depolarization
depolarization
repolarization
repolarization
0
5
85
50
125
130
145
150
190
225
250
600
P
P
P-Q
interval
QRS
T
0.05
0.8-1.0
0.8-1.0
0.02-0.05
1.0-1.5
3.0-3.5
-
0.8
(transverse)
0.5
70-80
20-40
*) Atrial repolarization occurs during the ventricular depolarization; therefore, it is not normally seen in the electrocardiogram.
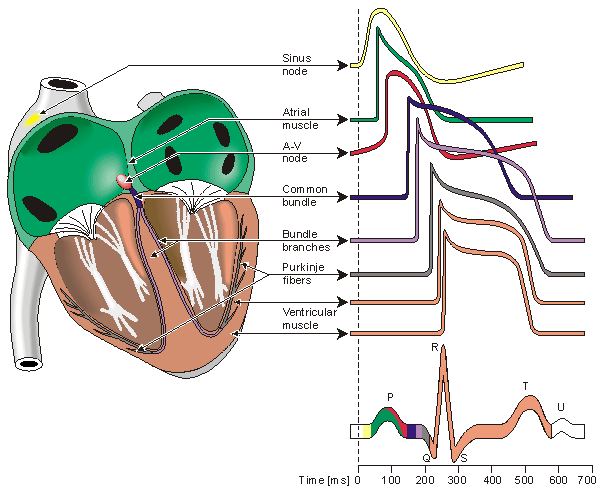
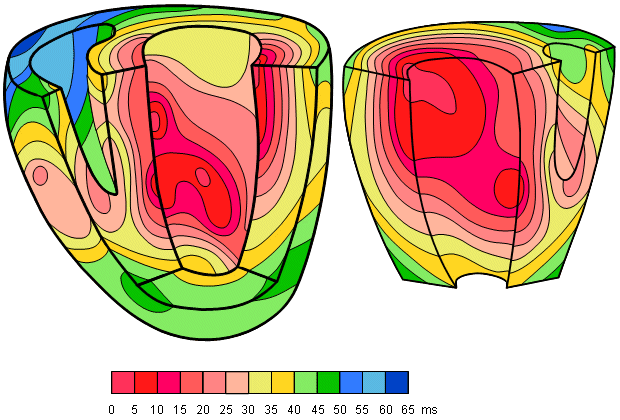
Fig. 6.8. Isochronic surfaces of the ventricular activation. (From Durrer et al., 1970.)
6.3 THE GENESIS OF THE ELECTROCARDIOGRAM
6.3.1 Activation Currents in Cardiac Tissue
Section 6.2.1 discussed cardiac electric events on an intracellular level. Such electric signals (as illustrated in Figs. 6.4, 6.5, and 6.7) may be recorded with a microelectrode, which is inserted inside a cardiac muscle cell. However, the electrocardiogram (ECG) is a recording of the electric potential, generated by the electric activity of the heart, on the surface of the thorax. The ECG thus represents the extracellular electric behavior of the cardiac muscle tissue. In this section we explain the genesis of the ECG signal via a highly idealized model.
 Figure 6.9A and B show a segment of cardiac tissue through which propagating depolarization (A) and repolarization (B) wavefront planes are passing. In this illustration the wavefronts move from right to left, which means that the time axis points to the right. There are two important properties of cardiac tissue that we shall make use of to analyze the potential and current distribution associated with these propagating waves. First, cells are interconnected by low-resistance pathways (gap junctions), as a result of which currents flowing in the intracellular space of one cell pass freely into the following cell. Second, the space between cells is very restrictive (accounting for less than 25% of the total volume). As a result, both intracellular and extracellular currents are confined to the direction parallel to the propagation of the plane wavefront.
Figure 6.9A and B show a segment of cardiac tissue through which propagating depolarization (A) and repolarization (B) wavefront planes are passing. In this illustration the wavefronts move from right to left, which means that the time axis points to the right. There are two important properties of cardiac tissue that we shall make use of to analyze the potential and current distribution associated with these propagating waves. First, cells are interconnected by low-resistance pathways (gap junctions), as a result of which currents flowing in the intracellular space of one cell pass freely into the following cell. Second, the space between cells is very restrictive (accounting for less than 25% of the total volume). As a result, both intracellular and extracellular currents are confined to the direction parallel to the propagation of the plane wavefront.
 The aforementioned conditions are exactly those for which the linear core conductor model, introduced in Section 3.4, fully applies; that is, both intracellular and extracellular currents flow in a linear path. In particular when using the condition Ii + Io = 0 and Equations 3.41
The aforementioned conditions are exactly those for which the linear core conductor model, introduced in Section 3.4, fully applies; that is, both intracellular and extracellular currents flow in a linear path. In particular when using the condition Ii + Io = 0 and Equations 3.41
  | (3.41) |
one obtains
 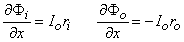 | (6.1) |
Integrating from x = – 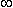 , to x = x gives
, to x = x gives
 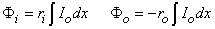 | (6.2) |
Subtracting the second of Equations 6.2 from the first and applying Vm = Fi - Fo, the definition of the transmembrane potential, we obtain:
 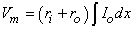 | (6.3) |
From Equation 6.3 we obtain the following important relationships valid for linear core conductor conditions, namely that
  | (6.4) |
and
 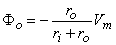 | (6.5) |
These equations describe "voltage divider" conditions and were first pointed out by Hodgkin and Rushton (1946). Note that they depend on the validity of Equation 3.36 which, in turn, requires that there be no external (polarizing) currents in the region under consideration.
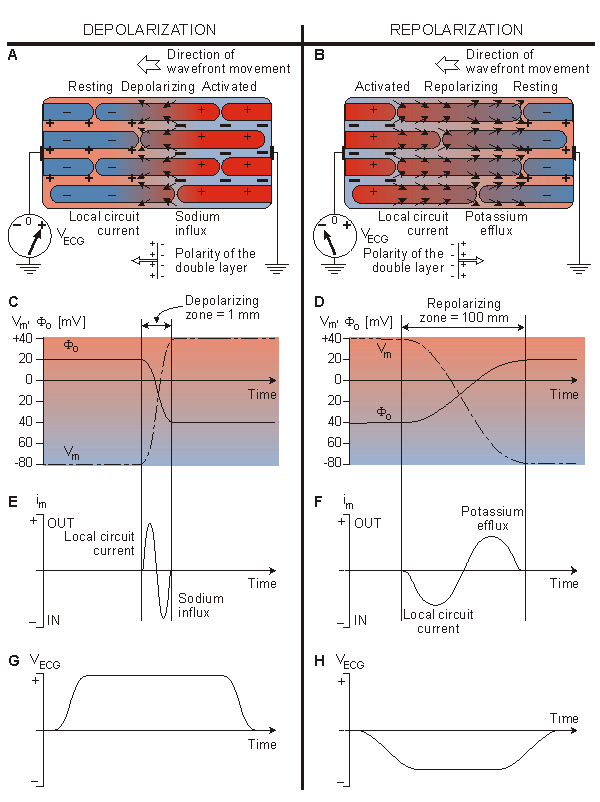
Fig. 6.9. The genesis of the electrocardiogram.
6.3.2 Depolarization Wave
We may now apply Equation 6.5 to the propagating wave under investigation. The variation in the value of Vm(x) is easy to infer from Figure 6.9C (dashed line) since in the activated region it is at the plateau voltage, generally around +40 mV, whereas in the resting region it is around -80 mV. The transition region is usually very narrow (about 1 mm, corresponding to a depolarization of about 1 ms and a velocity < 1 m/s), as the figure suggests. Application of Equation 6.4 results in the extracellular potential (Fo) behavior shown in Figure 6.9C (solid line). In Figure 6.9, the ratio ro/(ro + ri) = 0.5 has been chosen on the basis of experimental evidence for propagation along the cardiac fiber axis (Kléber and Riegger, 1986).
 The transmembrane current Im can be evaluated from Vm(x) in Figure 6.9C by applying the general cable equation (Equation 3.45):
The transmembrane current Im can be evaluated from Vm(x) in Figure 6.9C by applying the general cable equation (Equation 3.45):
 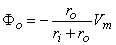 | (3.45) |
The equation for the transmembrane current im is thus
 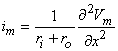 | (6.6) |
This current is confined to the depolarization zone. As shown in Figure 6.9A, just to the right of the centerline it is inward (thick arrows), and just to the left it is outward (thin arrows). The inward portion reflects the sodium influx, triggered by the very large and rapid rise in sodium permeability. The current outflow is the "local circuit" current which initially depolarizes the resting tissue, and which is advancing to the left (i.e., in the direction of propagation). The course of the transmembrane current is approximated in Figure 6.9E using Equation 6.6.
 An examination of the extracellular potential Fo shows it to be uniform except for a rapid change across the wavefront. Such a change from plus to minus is what one would expect at a double layer source where the dipole direction is from right to left (from minus to plus as explained in Section 11.2). So we conclude that for the depolarization (activation) of cardiac tissue a double layer appears at the wavefront with the dipole orientation in the direction of propagation. One can also approximate the source as proportional to the transmembrane current - estimated here by a lumped negative point source (on the right) and a lumped positive point source (on the left) which taken together constitute a dipole in the direction of propagation (to the left).
An examination of the extracellular potential Fo shows it to be uniform except for a rapid change across the wavefront. Such a change from plus to minus is what one would expect at a double layer source where the dipole direction is from right to left (from minus to plus as explained in Section 11.2). So we conclude that for the depolarization (activation) of cardiac tissue a double layer appears at the wavefront with the dipole orientation in the direction of propagation. One can also approximate the source as proportional to the transmembrane current - estimated here by a lumped negative point source (on the right) and a lumped positive point source (on the left) which taken together constitute a dipole in the direction of propagation (to the left).
 Finally, a double layer, whose positive side is pointing to the recording electrode (to the left), produces a positive (ECG) signal (Figure 6.9G).
Finally, a double layer, whose positive side is pointing to the recording electrode (to the left), produces a positive (ECG) signal (Figure 6.9G).
6.3.3 Repolarization Wave
The nature of the repolarization wave is in principle very different from that of the depolarization wave. Unlike depolarization, the repolarization is not a propagating phenomenon. If we examine the location of repolarizing cells at consecutive time instances, we can, however, approximate the repolarization with a proceeding wave phenomenon. As stated previously, when a cell depolarizes, another cell close to it then depolarizes and produces an electric field which triggers the depolarization phenomenon. In this way, the depolarization proceeds as a propagating wave within cardiac tissue.
As stated previously, when a cell depolarizes, another cell close to it then depolarizes and produces an electric field which triggers the depolarization phenomenon. In this way, the depolarization proceeds as a propagating wave within cardiac tissue.
 Repolarization in a cell occurs because the action pulse has only a certain duration; thus the cell repolarizes at a certain instant of time after its depolarization, not because of the repolarization of an adjoining cell. If the action pulses of all cells are of equal duration, the repolarization would of course accurately follow the same sequence as depolarization. In reality, however, this is not the case in ventricular muscle. The action pulses of the epicardial cells (on the outer surface) are of shorter duration than those of the endocardial cells (on the inner surface). Therefore, the "isochrones" of repolarizing cells proceed from the epicardium to the endocardium, giving the illusion that the repolarization proceeds as a wave from epicardium to endocardium.
Repolarization in a cell occurs because the action pulse has only a certain duration; thus the cell repolarizes at a certain instant of time after its depolarization, not because of the repolarization of an adjoining cell. If the action pulses of all cells are of equal duration, the repolarization would of course accurately follow the same sequence as depolarization. In reality, however, this is not the case in ventricular muscle. The action pulses of the epicardial cells (on the outer surface) are of shorter duration than those of the endocardial cells (on the inner surface). Therefore, the "isochrones" of repolarizing cells proceed from the epicardium to the endocardium, giving the illusion that the repolarization proceeds as a wave from epicardium to endocardium.
 If the cardiac action pulse were always of the same shape, then following propagation of depolarization from right to left, the recovery (repolarization) would also proceed from right to left. This case is depicted in the highly idealized Figure 6.9B, where the cells that were activated earliest must necessarily recover first. The recovery of cardiac cells is relatively slow, requiring approximately 100 ms (compare this with the time required to complete activation - roughly 1 ms). For this reason, in Figure 6.9B we have depicted the recovery interval as much wider than the activation interval.
If the cardiac action pulse were always of the same shape, then following propagation of depolarization from right to left, the recovery (repolarization) would also proceed from right to left. This case is depicted in the highly idealized Figure 6.9B, where the cells that were activated earliest must necessarily recover first. The recovery of cardiac cells is relatively slow, requiring approximately 100 ms (compare this with the time required to complete activation - roughly 1 ms). For this reason, in Figure 6.9B we have depicted the recovery interval as much wider than the activation interval.
 The polarity of Vm(x) decreases from its plateau value of +40 mV on the left to the resting value of -80 mV on the right (Figure 6.9D (dashed line)). Again, Equation 6.5 may be applied, in this case showing that the extracellular potential Fo (solid line) increases from minus to plus. In this case the double layer source is directed from left to right. And, it is spread out over a wide region of the heart muscle. (In fact, if activation occupies 1 mm, then recovery occupies 100 mm, a relationship that could only be suggested in Figure 6.9B, since in fact, it encompasses the entire heart!)
The polarity of Vm(x) decreases from its plateau value of +40 mV on the left to the resting value of -80 mV on the right (Figure 6.9D (dashed line)). Again, Equation 6.5 may be applied, in this case showing that the extracellular potential Fo (solid line) increases from minus to plus. In this case the double layer source is directed from left to right. And, it is spread out over a wide region of the heart muscle. (In fact, if activation occupies 1 mm, then recovery occupies 100 mm, a relationship that could only be suggested in Figure 6.9B, since in fact, it encompasses the entire heart!)
 The transmembrane current Im can be again evaluated from Vm(x) in Figure 6.9D by applying Equation 6.6. As shown in Figure 6.9B, to the right of the centerline it is outward (thick arrows) and just to the left it is inward (thin arrows). The outward portion reflects the potassium efflux due to the rapid rise of potassium permeability. The current inflow is again the "local circuit" current. The course of the transmembrane current during repolarization is approximated in Figure 6.9F.
The transmembrane current Im can be again evaluated from Vm(x) in Figure 6.9D by applying Equation 6.6. As shown in Figure 6.9B, to the right of the centerline it is outward (thick arrows) and just to the left it is inward (thin arrows). The outward portion reflects the potassium efflux due to the rapid rise of potassium permeability. The current inflow is again the "local circuit" current. The course of the transmembrane current during repolarization is approximated in Figure 6.9F.
 Thus, during repolarization, a double layer is formed that is similar to that observed during depolarization. The double layer in repolarization, however, has a polarity opposite to that in depolarization, and thus its negative side points toward the recording electrode; as a result, a negative (ECG) signal is recorded (Figure 6.9H).
Thus, during repolarization, a double layer is formed that is similar to that observed during depolarization. The double layer in repolarization, however, has a polarity opposite to that in depolarization, and thus its negative side points toward the recording electrode; as a result, a negative (ECG) signal is recorded (Figure 6.9H).
 In real heart muscle, since the action potential duration at the epicardium is actually shorter than at the endocardium, the recovery phase appears to move from epicardium to endocardium, that is, just the opposite to activation (and opposite the direction in the example above). As a consequence the recovery dipole is in the same direction as the activation dipole (i.e. reversed from that shown in Figure 6.9B). Since the recovery and activation dipoles are thus in the same direction one can explain the common observation that the normal activation and recovery ECG signal has the same polarity..
In real heart muscle, since the action potential duration at the epicardium is actually shorter than at the endocardium, the recovery phase appears to move from epicardium to endocardium, that is, just the opposite to activation (and opposite the direction in the example above). As a consequence the recovery dipole is in the same direction as the activation dipole (i.e. reversed from that shown in Figure 6.9B). Since the recovery and activation dipoles are thus in the same direction one can explain the common observation that the normal activation and recovery ECG signal has the same polarity..
REFERENCES
Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC (1970): Total excitation of the isolated human heart. Circulation 41:(6) 899-912.
Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HI (eds.) (1991): The Heart and Cardiovascular System, 2193 pp. Raven Press, New York.
Hodgkin AL, Rushton WA (1946): The electrical constants of a crustacean nerve fiber. Proc. R. Soc. (Biol.) B133: 444-79.
Kléber AG, Riegger CB (1986): Electrical constants of arterially perfused rabbit papillary muscle. J. Physiol. (Lond.) 385: 307-24.
Netter FH (1971): Heart, Vol. 5, 293 pp. The Ciba Collection of Medical Illustrations, Ciba Pharmaceutical Company, Summit, N.J.
Ruch TC, Patton HD (eds.) (1982): Physiology and Biophysics, 20th ed., 1242 pp. W. B. Saunders, Philadelphia.
Williams PL, Warwick R (eds.) (1989): Gray's Anatomy, 37th ed., 1598 pp. Churchill Livingstone, Edinburgh.
REFERENCES, BOOKS
Hurst JW, Schlant RC, Rackley CE, Sonnenblick EH, Wenger NK (eds.) (1990): The Heart: Arteries and Veins, 7th ed., 2274 pp. McGraw-Hill, New York.
Macfarlane PW, Lawrie TDV (eds.) (1989): Comprehensive Electrocardiology: Theory and Practice in Health and Disease, 1st ed., Vols. 1, 2, and 3, 1785 pp. Pergamon Press, New York.


