Research & Peer Review Studies
Kenrico is committed to cruelty-free science. We strictly oppose animal testing and ensure the safety and efficacy of our products through ethical clinical evaluations.
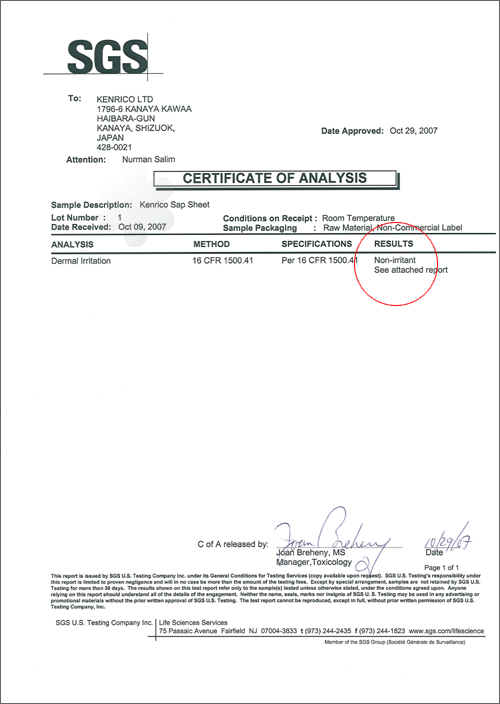
SOP49: FHSA Primary Skin Irritation (March 3, 2008)
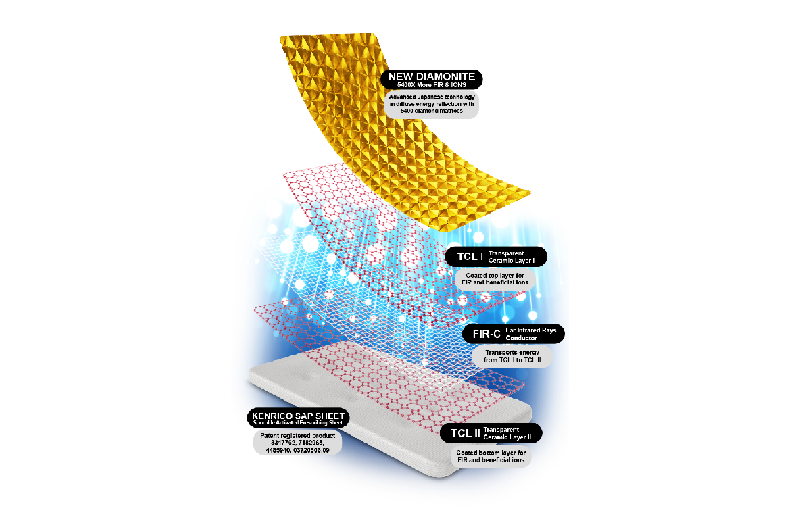
Test article: Kenrico SAP Sheet
Test devices: Micropore surgical tape, dental dam/rubber sheeting, 25-gauge needle
This study evaluated the degree of skin irritation caused by the test article.
Result: Non-irritant
SOP49: Microbial Test (March 3, 2008)
Test article: Kenrico SAP Sheet
Test devices: TSA, SDA, TSB-M, LCB-M, SC, TTB with BG, BP, CA, MAC, XLD, BGA, BSA
This procedure estimates the number of viable aerobic microorganisms in the product and ensures it's free from designated microbial species.
Result: Free from germs and bacteria
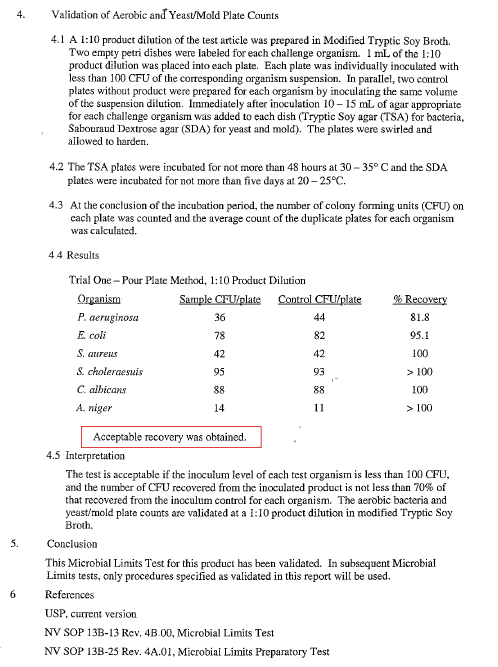
SOP49: Microbial Limit Test (June 26, 2003)
Test article: Kenrico SAP Sheet
Test devices: TSA, SDA, TSB-M, LCB-M, SC, TTB with BG, BP, CA, MAC, XLD, BGA, BSA
Performed on raw and finished pharmaceutical products to determine viable aerobic microorganisms and ensure safety from targeted pathogens.
SOP49: USP Microbial Limit Validation (June 9, 2003)
Test article: Kenrico SAP Sheet
Test devices: TSA, SDA, TSB, LCB, SC, TTB, MAC, BP, CA, XLD, BGA, BSA
This validation confirms the effectiveness of the microbial limit procedures and ensures accurate detection of microbial contamination across multiple sample types.
Result: Safe and free from microorganisms
Microbial Limit Validation
-
NEURO47: THETA BRAINWAVES
March 3 2025
-
CTMED575: CT SCAN DIAGNOSIS WITH X-RAYS
October 1 2022 - December 1 2022
-
NERVES & LIGAMENTS: TRMX LIFE PLUS
January 15 2021
-
3D NEUROIMAGING: TRMX 4 ORIGINAL
July 17 2018
-
EEG AND ALPHA: TRMX-3 30th ANNIVERSARY
July 4 2014 - February 14 2015
-
FAT AND CELLULITE RESEARCH OF DIAMOND HYBRID EDITION DHE
June 2 2013 - July 11 2013
-
NATURAL ENERGY RESEARCH OF DIAMOND HYBRID EDITION DHE
October 27 2013
-
DEFENSOR II STUDY OF TRMX-3
May, 2011 - February 28, 2013
-
LEXIRIN PADS AS ELIXIR OF LIFE
October 1 2005 - September 1 2012
-
TRMX-3 BOOSTS MENTAL AND PHYSICAL HEALTH
February 1 2011 - March 1 2011
-
ZEO REMOVES HEAVY METALS
December 5 2008 - March 6 2009
-
MICROSCOPIC RESEARCH
June 11 2008
-
LIFESTYLE AND BLOOD ANALYSIS
October 25 2006 - April 25 2007
-
LDL CHOLESTEROL AND TRIGLYCERIDES REDUCED
May 30 2006
-
HEAVY METALS RESEARCH (ADDITIONAL REPORT)
October 7 2005
-
HEAVY METALS RESEARCH
January 18 2005
-
NEGATIVE IONS RESEARCH
June 14 2004
-
FAR INFRARED RESEARCH
June 3 2004
-
BRAIN WAVE RESEARCH
June 6 2003
-
QUALITY & SAFETY TESTING