Research & Peer Review Studies
By partnering with state-of-the-art research laboratories in USA (USP, FDA level laboratory) and Japan, we can continue to invest in the research and innovation of alternative medicines. This research enables us to provide, what we believe, are the best and safest products on the market to our clients, today and in years to come.
The following research information applies only for products manufactured by Kenrico. Any other use of the research information including translation in other languages, without written permission of Kenrico is strictly prohibited and subjected to severe penalty. By viewing the research information under this paragraph, you have agreed to the Terms of Use.
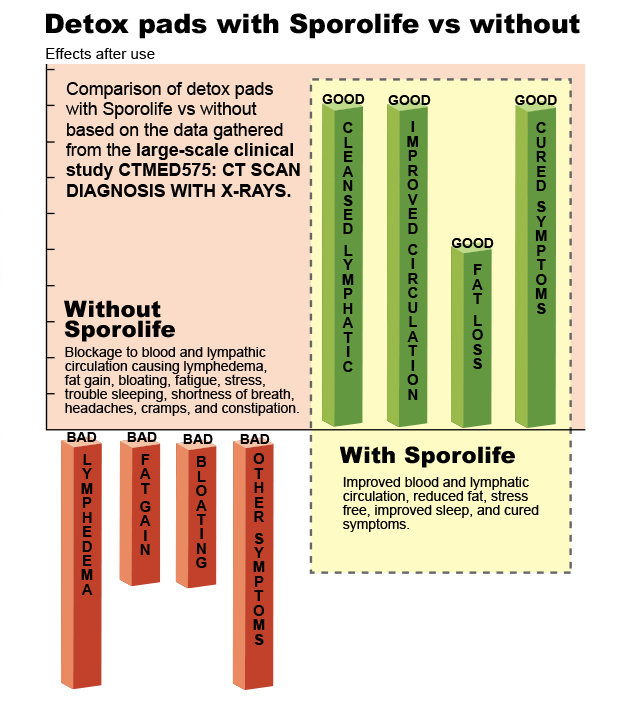
1. REVEALING THE SECRET OF DETOX PADSCT SCAN DIAGNOSIS WITH X-RAYS
A large scale single blind clinical study utilizing CT scan to explore the effect of Sporolife in changing the blood and lymphatic circulation in human body.
RESULT:
Clinical study confirmed the mandatory requirement of Sporolife in the product by the European Authority. In the clinical trial, Sporolife was able to demonstrate its positive effect in
improving health by reducing fat percentage up to 6% without exercising including curing many health symptoms such as lymphedema (bloating), fatigue, shortness of breath, headaches, cramps, and constipation. The curing effect is strongly related
to the improved circulation in the blood and lymphatic system.
Based on the validation of results from the current human clinical trial, natural compositions that do not contain Sporolife, is responsible for weight gain, body fat increase, and life-threatening injuries.
2. NERVES AND LIGAMENTS
A clinical study to investigate the effects of TRMX LIFE PLUS on the meridian nerve and transverse carpal ligaments in 1000 subjects with various degree of carpal tunnel syndrome.
RESULT: 86.40% of patients reported full recovery with 5% of patients and 8.60% of patients reported vast improvement to their condition on the 6th week.
3. 3D NEUROIMAGING WITH EGG AND BLOOD ANALYSIS
A large scale single blind clinical study (200 participants) to investigate the effectiveness of Kenrico Supreme Gold Edition TRMX 4 Original.
RESULT: Sporolife was found to be an essential composition to eliminate lymphedema with symptoms of weight gain, bloating, poisoning, and elevated stress or depression.
4. EEG BRAINWAVES SYNCH AND ALPHA BRAINWAVES COMPARISON
A large scale single blind clinical study (200 participants) to investigate the effectiveness of Kenrico Supreme Gold Edition (TRMX-3 30th Anniversary).
RESULT: 19% increase of total alpha brainwaves, 136 nV increase of maximum alpha brainwaves, 11% increase of Sorgi-Einstein index.
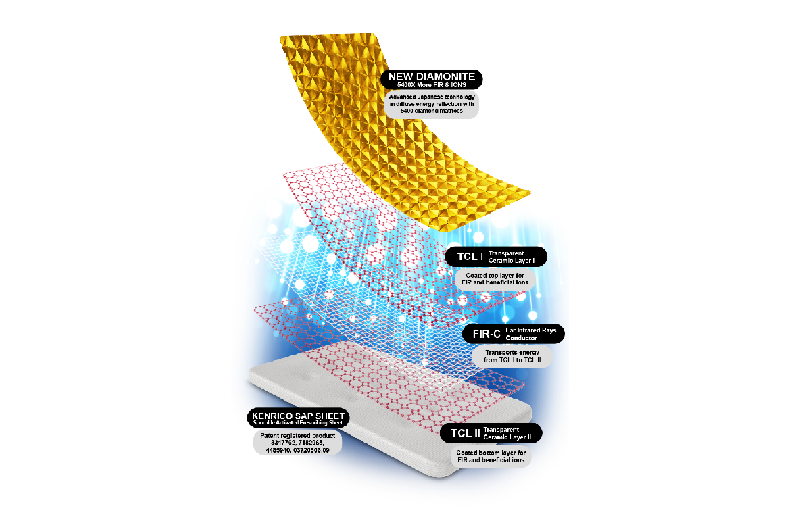
5. FAT AND CELLULITE RESEARCH OF DIAMOND HYBRID EDITION (DHE)
An open trial clinical study examines the effectiveness of The New Diamond Hybrid Edition (DHE) for cleansing toxins and for burning excess fats and cellulite while the body is at rest.
RESULT: The Diamond Hybrid Edition (DHE ®) eliminates excess fats and cellulite (overall -15%, up to -10% on abdomen and -11.3% on legs) within 30 days.
6. NATURAL ENERGY RESEARCH OF DIAMOND HYBRID EDITION (DHE)
Third-party independent laboratory analysis to inspect the medical engineering and to examine the effectiveness of DHE to combat diseases.
RESULT: The Diamond Hybrid Edition (DHE ®) provide the HIGHEST amount of beneficial energy to eliminate toxic accumulation in the body, enlarged fat cells, water retention, fibrosis and all fat-related diseases.
7. DEFENSOR II STUDY OF TRMX-3
DEFENSOR II, a phase 4 human clinical trial, examines the effectiveness of cures utilizing TRMX-3 for physical & mental stress, depression, heavy metals poisoning, inflammation and pain.
RESULT: The Supreme Gold Edition (TRMX-3 ®) provide powerful effects in boosting health when administered as a short-term or a long-term treatment.
8. LEXIRIN PADS AS ELIXIR OF LIFE
Human clinical trial with 300 participants to investigate the effectiveness of The Superior Edition (LEXIRIN) ®.
RESULT: Clinical trial confirmed the positive effects of the Superior Edition (LEXIRIN) in treating gastrointestinal disorders and other wide range of diseases including Alzheimer's disease. LEXIRIN also protects organs and can be used as elixir of life to increase the human lifespan.
9. TRMX-3 BOOSTS MENTAL AND PHYSICAL HEALTH
Human clinical trial conducted by third-party independent medical clinic, Woking Football Club, United Kingdom.
RESULT: a maximum of 300% of improved performance and winning percentages during the clinical trial with the TRMX-3 ®.
10. ZEO REMOVES HEAVY METALS
Clinical study conducted by third-party independent research center, Japan Hikari Research Institute.
RESULT: Significant removal of heavy metals from the body after 3 months of continuous use.
11. MICROSCOPIC RESEARCH
Analysis conducted by third-party independent laboratory, JFR Laboratories to investigate the efficacy of toxins removal utilizing Kenrico Sap Sheet or Detox Pads.
RESULT: Large amount of toxins including heavy metals, excess uric acid, excess oxalic acid are successfully removed.
12. LIFESTYLE AND BLOOD ANALYSIS
Clinical study through complete blood test conducted by third-party independent research center, Japan Hikari Research Institute.
RESULT: Noticeable to significant improvement in liver and kidney function, and completely reduced the threat of developing diabetes, gout and infection.
13. LDL CHOLESTEROL AND TRIGLYCERIDES REDUCED
Clinical study conducted by third-party independent research center and laboratory, Japan Hikari Research Institute, Research Testing Institute and Core Laboratory in USA.
RESULT: Superb reduction to LDL-cholesterol and excess triglycerides to all participants that have borderline to high cholesterol levels.
14. HEAVY METALS RESEARCH
(ADDITIONAL REPORT)
Additional report from Japan Hikari Research Institute.
15. HEAVY METALS RESEARCH
Clinical study conducted by third-party independent research center, Japan Hikari Research Institute.
RESULT: Successful removal of ALL heavy metals from the body after continuous 3 months of detoxification using Kenrico Sap Sheet or Detox Pads.
16. NEGATIVE IONS RESEARCH
Third-party independent analysis conducted by Far Infrared Research Organization.
RESULT: Kenrico Sap Sheet emits the highest amount of negative ions.
17. FAR INFRARED RESEARCH
Third-party independent analysis conducted by Far Infrared Research Organization.
RESULT: Improved blood circulation and evidence of far infrared rays from the Kenrico Sap Sheet or Detox Pads. Far infrared originated from Kenrico Sap Sheet is 100% natural & 100% safe, made from natural ingredients, emitted without the help of electric devices.
18. BRAIN WAVE RESEARCH
Clinical study conducted by third-party independent research center, Far Infrared Research Organization.
RESULT: Kenrico Sap Sheet significantly increases alpha brainwave. Alpha brainwaves are conducive to creative problem solving, accelerated learning, mood elevation and stress reduction.
19. QUALITY & SAFETY TESTING
Complete analysis by state-of-the-art research laboratories to test the safety and the quality of Kenrico products.
RESULT: Absolutely free (100% free & safe) of microorganism, germs and pathogens. 100% free of irritant and allergic compounds.