Prescribing Information
For Healthcare Professionals
The product information presented here has been approved by Japanese Ministry of Health. Other countries may have different regulatory requirements and review practices that may require referencing different information.
KENRICO SAP SHEET ®
MEGA GOLD SDX1, MEGA ZEO, TRMX 5 50TH ANNIVERSARY, TRMX LIFE PLUS, TRMX LIFE, AGi, FX-2, EXA-2i, KX-2, SS1Li, SS2Li, SS3Li, GPi, TG-1i, QU-1, CCi, WRX-2i 30TH ANNIVERSARY, AQUA COOL TUV, ENHANCED AQUA COOL TUV, LEXIRIN
DESCRIPTION
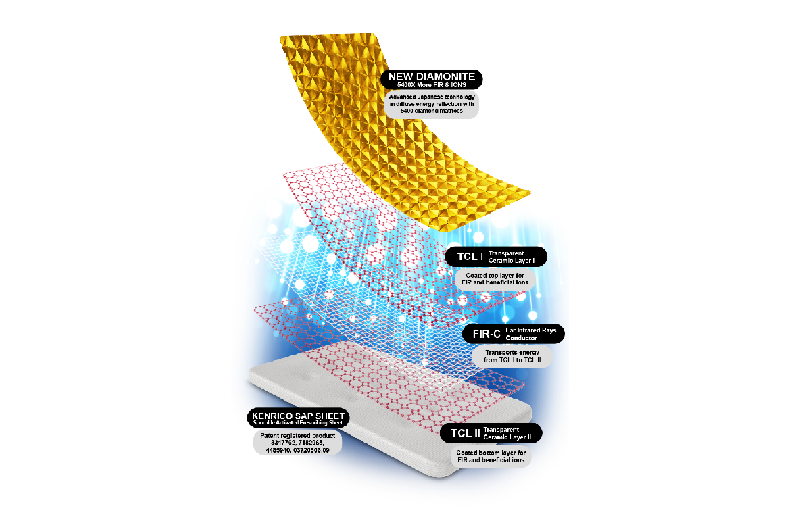
Kenrico SAP Sheet is supplied as pad, for skin surface administration. Each pad contains 4000, 5000, 8500, 9000 mg, 17000 mg, and 23000 mg of patented formulation with Sporolife®, ZeoCell, Diamonite®, Hydrogen Antioxidant, and Holomedic.
APPLICATION INSTRUCTIONS
Link to application instruction for MEGA GOLD SDX1, MEGA ZEO, TRMX 5 50TH ANNIVERSARY, AGi, FX-2, EXA-2i, KX-2, SS1Li, SS2Li, SS3Li, GPi, TG-1i, QU-1, CCi, WRX-2i 30TH ANNIVERSARY, AQUA COOL TUV, ENHANCED AQUA COOL TUV, LEXIRIN.
Link to application instruction for TRMX LIFE.
Link to application instruction for TRMX LIFE PLUS.
CLINICAL PHARMACOLOGY
Kenrico Sporolife Activated Prescribing Sheet or SAP Sheet (US Patent 8317962, European Patent EP2908807B1) promotes detoxification, blood circulation, and relaxation with sporolife. Most patients with emotional stress and depression treated with Kenrico SAP Sheet experience a substantial increase of alpha brain wave along with decreased stress, anxiety, depression and their associated headache, nausea, sweating and dizziness.
Sporolife ® is conducive to the function of Kenrico SAP Sheet. In all patients treated with Kenrico SAP Sheet impregnated with Sporolife ®, lymphedema were successfully treated and stress was eliminated and replaced with a substantial increase in relaxation.
Most patients with cardiovascular problems treated with Kenrico SAP Sheet experience decreased frequency and severity of hypertension attacks. In patients who respond, blood pressure stabilizes progressively during the first two days of therapy with Kenrico SAP Sheet; after withdrawal, blood pressure usually increases slightly below pre-treatment level within two to three days.
In patients with elevated LDL and Triglycerides, who produce limited insulin, administration of four to six pads of Kenrico SAP Sheet per day has reduced elevated LDL and Triglycerides from about 35 to 80 percent as measured by the total cholesterol level and their metabolites. The maximum biochemical effect usually occurs within nine to ten days, and the level of LDL and Triglycerides usually remains to post-treatment levels within three to four days after Kenrico SAP Sheet is discontinued. In some patients the level of LDL and Triglycerides may be balanced to normal or near normal levels. In most patients the duration of the treatment has been twelve to thirteen weeks, but several patients have received Kenrico SAP Sheet for periods of one to 2 years. Similar positive effects are also reported in patients with liver problems.
Kenrico SAP Sheet function well from the foot soles. Hair analysis and blood analysis techniques presented extraction of heavy metals after 3 months therapy. The quantities extracted are sufficient to determine accurate determination of blood analysis by routine techniques. Most patients undergo treatment with Kenrico SAP Sheet experience significant improvement during pre and post treatment from the symptoms and the episodes with the addition of Kenrico Carbon-Titanium Adhesives.
INDICATIONS AND USAGE
Consultation to KenricoCare is encouraged for patients with health complications to obtain weekly schedule for the treatment. KenricoCare is operated by licensed medical practitioners and is available 24 hours a day, 7 days a week. It is managed according to the requirement of ISO 9001:2008 Quality Management System.
MEGA GOLD EDITION SDX1 17000 mg ®
The extraordinary curing potential of MEGA GOLD EDITION SDX 1 17000 mg, or popularly known as the Mega Gold, stems from its dosage of 17000 mg and 5000 mg of SPOROLIFE ® plus natural Hydrogen Antioxidant.
Medical data indicates that the Mega Gold provides faster relief with three times the effectiveness of the Supreme Gold Edition TRMX 5 50th Anniversary 8500 mg.
It is the largest pad available, with massive dimensions of 120 x 90 mm, and the heaviest composition, weighing 17000 mg.
Recommended for alleviating symptoms that require quick attention, such as severe fatigue, headaches, double vision, blood pressure issues, arthritis, rheumatism, skin problems, stress, slow learning, menopause-related hot flashes, mood swings, Crohn's disease, fibromyalgia, and heavy metal poisoning.
Intended for patients with chronic illnesses, diseases, and disorders.
MEGA ZEO ®
ZeoCell is a natural, highly effective formulation designed to support cellular repair and anti-aging treatment. As the core component of MEGA ZEO, ZeoCell works synergistically within the body to promote cellular rejuvenation and regeneration.
The MEGA ZEO has a massive dimension of 120 x 90 mm and contains a total composition of 17000 mg consisting of Sporolife, ZeoCell, diatomaceous earth, tourmaline, aloe vera, ginger and Arnica.
The use of MEGA ZEO is associated with improvements in tissue repair rates and reductions in oxidative stress markers. Due to its regenerative properties, MEGA ZEO is beneficial for patients seeking cellular repair from environmental or age-related cellular degradation.
MEGA ZEO is recommended for patients experiencing heavy metal toxicity, acute and chronic injuries (internal and external), and conditions associated with accelerated cellular aging.
SUPREME GOLD EDITION TRMX 5 50TH ANNIVERSARY ®
The TRMX 5 50TH ANNIVERSARY contains a dosage of 8500 mg and formulated with 855 mg of SPOROLIFE ®, natural Hydrogen Antioxidant and DIAMONITE ®. Utilizes the latest medical technology of 5400 individually constructed diamond shapes to provide superior detoxification, strongest analgesic, anti-inflammation, and anti-stress properties. Aid in detoxing heavy metals, in boosting intelligence, and in restoring vitality.
TRMX 5 50TH ANNIVERSARY marked the 50th years of combined effort between Kenrico and healthcare professionals in changing lives. A large-scale clinical study conducted in 2017 has confirmed the effectiveness of the product in increasing Sorgi-Einstein or SE index. Productivity, intelligence, and quality of life are referred to as Sorgi-Einstein index or SE index.
TRMX 5 50TH ANNIVERSARY is recommended for these symptoms: Fatigue, headache, brain fog, slow learning, double vision, blood pressure, arthritis, rheumatism, skin problems, stress, hot flashes due to menopause, mood swings, Crohn's disease, Fibromyalgia, and severe heavy metal poisoning.
TRMX 5 50TH ANNIVERSARY is intended for patients that require immediate attention and immediate relief of the symptoms.
SUPREME GOLD EDITION TRMX LIFE PLUS ®
TRMX LIFE PLUS is a self-stick pad with natural Sporolife that focus on relieving pain and discomfort in the jaws, hands, wrists, fingers, and arms. It can also relief joint stiffness by significantly improve the flexibility of the joints. TRMX LIFE PLUS has been verified effective for treating Carpal Tunnel Syndrome (CTS) by the latest clinical study.
TRMX LIFE PLUS is individually protected and naturally preserved by a new antigerm packaging that blocks the outside air and germs from entering the product. The product has zero degradation and is guaranteed 100% fresh upon delivery.
TRMX LIFE is highly recommended by Harvard doctor for treating pain, inflammation, numbness, and joint stiffness in the jaws, hands, fingers, and arms. It is effective for treating Carpal Tunnel Syndrome and TMJ (Temporomandibular Joint) disorder.
SUPREME GOLD EDITION TRMX LIFE ®
TRMX LIFE is the first hybrid pad for both the feet and the hands. It has self-stick technology and contains natural Sporolife that provides 25x more analgesic (anti-pain), 25x more anti-inflammation, 25x more anti-stress, 25x more cleansing, and 25x more positive mental state boosting capability. For feet application, its built-in adhesive strips enable ease of use with any socks. For hand application, its built-in adhesive strips enable the use as is without the need of any adhesive sheets.
TRMX LIFE is highly recommended by Harvard doctor and by other medical professionals for treating illnesses, diseases, and disorders. TRMX LIFE is recommended for curing these conditions: heavy metals poisoning, chemical poisoning, germs infectious diseases, lymphatic disease, mental disorders, learning disability, and all types of arthritis that includes pain & discomfort in hands, wrists, and arms.
SILVER EDITION AGi ®
The key ingredients in the silver pads are Sporolife ABM ( Agaricus Blazei Murrill mushroom). AGi is recommended for these symptoms: Fatigue, headache, double vision, arthritis, low body immunity, hormone imbalances, acne, diabetes and allergies.
Agaricus Blazei is a species of mushroom, known as himematsutake and by a number of other names. This mushroom is best known for its purported medicinal properties. Because of its high beta glucan content, higher than both Reishi and Shiitake mushrooms, Agaricus Blazei is used in oncological therapy (branch of medicine that studies the development, diagnosis, treatment and prevention of tumor/cancer), mainly in Japan and California. It has been commercially cultivated in Asia and South America since 1993.
Because of this valuable polysaccharide, and lack of supply, Agaricus blazei used to be relatively expensive, until it was successfully artificially cultivated in mushroom farms. Agaricus blazei mushroom specifically assists in the production of interferon and interleukin, which are potent in fighting off cancer cell metastasis, especially cancer of the uterus. It also reduces blood glucose, blood pressure, cholesterol levels and the effects of arteriosclerosis.
ABM is known to contain three different beta- glucans - beta-(1-3)-D- glucan, beta-(1-4)-a -D- glucan, and beta-(1-6)-D- glucan.
BLUE EDITION FX-2 ®
The blue edition pads are special pads that contain Sporolife and a very high amount of antiseptic properties.
FX-2 is recommended for those who suffer from Fibromyalgia, Lyme disease and psoriasis. Also for symptoms of: fatigue, headache, double vision, blood pressure, arthritis, rheumatism, gout, and skin problems, hot flashes due to menopause, chronic sinusitis and allergies.
RED EDITION EXA-2i ®
The red edition pads contain Sporolife and organic cayenne. Cayenne pepper, also called Capsicum frutescens, is a stimulating herb made from the dried pods of chili peppers and is well known for its pungent taste and smell. Cayenne is used worldwide to treat a variety of health conditions, including poor circulation, weak digestion, heart disease, chronic pain, sore throats, headaches and toothache.
The key ingredient in the red edition pads is Sporolife and organic cayenne. EXA-2i is recommended for these symptoms: Fatigue, headache, sore throats, toothache, double vision, blood pressure, arthritis, rheumatism, gout, skin problems, weak digestion, and heart disease.
GREEN EDITION KX-2 ®
The green editions pads are the only pads in the world with Sporolife, tourmaline and amethyst. KX-2 the highest amount of far infrared and negative ions.
The key ingredients in the Green edition pads are Sporolife, tourmaline and amethyst. Amethyst increases the amount of negative ion and far infrared rays emitted from the pads. When pairing it with Tourmaline, Amethyst increases the amount of negative ion and far infrared rays by almost double (200%).
The GREEN KX-2 model emits negative ion on average of 3100 ion/cm3. The KX-2 is recommended for these symptoms: Fatigue, headache, double vision, blood pressure, arthritis, rheumatism, skin problems, stress, slow learning, hot flashes due to menopause, mood swings, Autism, Crohn's disease, fibromyalgia, and heavy metal poisoning.
GRAPEFRUIT EDITION SS1Li ®
In patients that are overweight, who responded to the treatment, administration of two pads of SS1Li per day has been able to promote further weight loss and reduce the risk of weight rebound.
The key ingredients in the grapefruit pads are Sporolife and organic grapefruit. SS1Li is recommended for these symptoms: overweight, acid reflux, weight loss, weight rebound. The grapefruit edition pad contains pectin, a soluble fiber that reduces the rate of entry of carbohydrates into your bloodstream, thereby lowering insulin secretion that could result in possible weight loss.
RASPBERRY EDITION SS2Li ®
The key ingredient in the raspberry pads is Sporolife and the organic raspberry. The SS2Li can strengthen the reproductive system. In females, it can strengthen the wall of the uterus to help a smooth delivery and is recommended during pregnancy. Known effective for these symptoms: Menstrual cramps, weak uterus wall, light to mild stress, fatigue, headache, double vision.
LAVENDER EDITION SS3Li ®
The key ingredient in the lavender pads is Sporolife and organic lavender. The SS3Li is made specifically to promote relaxation. Some user may experience improved body scent and the removal of body and body odor after use. The effect is caused by the high amount of lavender and vinegar in removing these bacterias.
BRONZE EDITION GPi ®
The GPi is made for users with skin allergies. The GPi contains Sporolife and vitamin C for detoxification and for improving skin condition. For users that are allergic to most prescription, the GPi provides an effective alternative method for the patients to detoxify without any adverse effect. Known effective for these symptoms: severe & chronic allergies, fatigue and headaches.
ENHANCED GRAPEFRUIT TG-1i ®
Enhanced grapefruit pads contain twice the amount of organic grapefruit in the regular organic grapefruit pads and intended for people with a severe overweight problems.
The enhanced grapefruit pads are made for weight loss. It contains a very high amount of pectin, a soluble fiber that reduces the rate of entry of carbohydrates into your bloodstream, thereby lowering insulin secretion. The key ingredients in the enhanced grapefruit pads are Sporolife and organic grapefruit.
QUICK EDITION QU-1 ®
Patients who are administered QU-1 continuously has reduced risk of liver infection and dysfunction.
The key ingredients in the QU-1 pads are Sporolife and organic milk Thistle. QU-1 is recommended for people suffering from liver dysfunction, liver infection, gallstones, inflammatory conditions (psoriasis) and for those who are recovering alcoholics. Milk Thistle protects the liver from damage and helps the liver make new healthy cells to replace those that have been damaged.
SPECIAL EDITION GREEN TEA CCi ®
EGCG (epigallocatechin-3-gallate), extracted from Japanese oldest green tea (Gyokuro) and Sporolife are the primary active ingredients in the CCi. It is known effective against cancer, tumor and can reduce the risk of cancer. EGCG can benefit joint health and bowel resistance to inflammation due to its anti-inflammatory characteristics, and may assist in weight loss since studies indicate that it can increase metabolic rate without side effects. CCi is recommended for these symptoms: high cholesterol and triglycerides, cancer, tumor, burns, itches, and skin condition.
HOT PINK EDITION WRX-2i 30TH ANNIVERSARY ®
Hot Pink Edition WRX-2i 30TH ANNIVERSARY contains Sporolife and organic cinnamon. Cinnamon is known to have insulin-like biological activity to improve blood glucose, triglyceride, total cholesterol, HDL cholesterol, LDL cholesterol levels in people with type 2 diabetes. In patients with type 1 diabetes that are dependent on insulin, the use of Hot Pink Edition WRX-2i 30TH ANNIVERSARY can improve the body condition and reduce the long-term side effects of insulin dependency such as hypoglycemia, weight gain and increased mortality rate.
Clinical study performed on Hot Pink Edition WRX-2i over six-month period has shown excellent result in treating diabetes, hepatitis, gout and infection with significant improvement of up to 50% in both glucose level and fat level.
SOOTHING EDITION AQUA COOL TUV ®
Aqua Cool TUV (Aqua Cool Therapeutic Ultramotivity Variant) emits soothing effects for curing fever, heat stroke, digestive problem, and excretory system problem. It utilizes Sporolife to provide a calming and relaxing sensation.
Aqua Cool TUV is recommended for these symptoms: heat exhaustion, heat stroke, sore throat, stomach problems, water retention, stress relief, IBS, hot flashes, mood swings, slow learning, pain, headache, digestive ulcers, stomach cramps, gas problems, constipation relief, inflammatory disorders, liver and gallbladder problems. Aqua Cool TUV has the capability to cleanse heavy metals at the same level of effectiveness as the TRMX-2i.
ENHANCED SOOTHING EDITION AQUA COOL TUV ®
Enhanced Aqua Cool TUV (Enhanced Aqua Cool Therapeutic Ultramotivity Variant) contains identical formulation as the original soothing edition with Sporolife, extra tourmaline and extra peppermint (Mentha piperita).
Enhanced Aqua Cool TUV is recommended for these symptoms: heat exhaustion, heat stroke, sore throat, stomach problems, water retention, stress relief, IBS, hot flashes, mood swings, slow learning, pain, headache, digestive ulcers, stomach cramps, gas problems, constipation relief, inflammatory disorders, liver and gallbladder problems.
SUPERIOR EDITION LEXIRIN ®
LEXIRIN has passed US FDA's Phase I human clinical trials at Alta Bates Medical Center in Berkeley. Facts indicate that cell damage (apoptosis) is prevalent in people with illnesses and aging and contributes to the gradual deterioration of the organ functions. LEXIRIN is made to prevent cell damage.
The LEXIRIN pad contains Sporolife, nattokinase, winter cherry (ashwagandha) and holy basil (tulsi). It prevents cell damage (apoptosis) and improves the condition of patients with gastrointestinal disorders, deleterious dermatological conditions, immunosuppression or immunodeficiency, reperfusion damage resulting from ischemia, cardiovascular disorders, wound healing, transplantation, tissue rejection or Alzheimer's disease.
CONTRAINDICATIONS
None
WARNINGS
None
PRECAUTIONS
Before use, carefully inspect the packaging label. If Sporolife or Kenrico is missing from the label, dispose of it immediately. The label must indicate Sporolife as part of its composition or identify Kenrico as the manufacturer. The use of a product lacking Sporolife may result in lymphedema, leading to symptoms such as weight gain, bloating, and depression: Neuroimaging & Blood Analysis and CT Scan Diagnosis with X-Rays. The European Authority mandates the inclusion of Sporolife in all skin pad products.
ADVERSE REACTIONS
None
OVERDOSAGE
At doses exceeding 24 pads/day, some degree of fatigue due to over-detox may occur. Reduction of the dosage results in the disappearance of these symptoms.
DOSAGE AND ADMINISTRATION
Optimal effective dosages of Kenrico SAP Sheet usually are between 2 to 8 pads per day, 4 to 12 hours per application, and the dose can be increased to maximum 12 pads per day by monitoring the clinical symptoms.