Corporate News
2025
KENRICO's NEURO47 Clinical Study Reveals Significant Increase in Theta Brainwaves.
March 12, 2025, Kenrico is proud to announce the findings of its latest clinical study, NEURO47, which demonstrates a substantial increase in theta brainwave activity among users of the Kenrico SAP Sheet.
An increase in theta wave activity is often associated with better sleep onset, improved dream states, and enhanced restorative sleep quality.
The study employed electroencephalography (EEG) to monitor brainwave patterns in participants using the Kenrico SAP Sheet. Results indicated a significant elevation in theta brainwave activity, suggesting that the product is an optimal solution for enhancing sleep quality.
"People struggling with sleep disorders such as insomnia, as well as those affected by PTSD, bipolar disorder, stress and depression, can now find relief with the Diamond GOLD and TRMX 5 50th Anniversary," said Watanabe M.D., Ph.D, deputy director and Kenrico senior head researcher.
"The key is in the Sporolife. The higher the dosage, the better the outcomes."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2024
Exciting Upgrade to the Longtime Bestseller: Kenrico Quick Edition QU-1.
December 19th, 2024, originally launched in 2004, the Kenrico QU-1 Quick Edition has been a trusted bestseller for nearly two decades. Now, it’s undergoing an exciting upgrade, with completion scheduled for January.
While retaining the iconic Quick Edition QU-1 name, the product now features a remarkable 5X increase in Sporolife content, from 200 mg to 1000 mg. Additionally, the new Kenrico Shield packaging, invented by Kenrico, offers superior protection.
This innovative packaging prevents air and germ contamination, ensures scents stay sealed, and maintains unparalleled freshness and quality. The Kenrico Shield replaces the conventional fiber packaging for a significant enhancement in product preservation.
"Kenrico continues to innovate and integrate advanced medical technologies for the benefit of our patients," said Nurman S., President of Kenrico.
"The increase in Sporolife and the introduction of Kenrico Shield ensure that our product remains effective, pure, and fresh."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Kenrico Factory Upgrade Boosts Quality and Efficiency.
October 1, 2024, Kenrico has successfully completed a significant upgrade to its factory, enhancing production capabilities.
Kenrico's original equipment is arranged sequentially to form a streamlined production line. From October 1st, Kenrico has successfully increased production lines from several shared lines for all models to exclusive lines dedicated to each model including the Mega Gold and Mega ZEO.
This significant increase in production lines will improve overall quality in terms of speed, efficiency, and reliability.
"Kenrico remains dedicated to delivering safe and effective products" said Nurman S, the President of Kenrico. "Our recent investment in upgrading our facilities will ensure continued quality and efficiency, allowing our clients to rely on us for many years to come."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Mega ZEO with ZeoCell In Production
June 25, 2024, Kenrico has announced the upgrade of its regular ZEO to the new Mega ZEO with ZeoCell, in response to growing demand.
"Following the success of Mega Gold SDX1, users have requested more larger pads to expand the scope of treatment," said Watanabe M.D., Ph.D, deputy director and Kenrico senior head researcher. "Mega ZEO is our answer to this demand. With its enhanced capabilities utilizing ZeoCell,
more conditions can be improved, more illnesses cured, and more people will be able to live healthier, fuller lives."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2023
Mega Gold Edition SDX1: A Revolution in Wellness.
October 26, 2023, Kenrico has announced an upgrade in the composition of SDX 1, increasing it from 16000 mg to 17000 mg.
In the world of health and wellness, groundbreaking innovations are rare, and even rarer are those that redefine history. With a groundbreaking 17000 mg of patented formula, the Mega Gold Edition SDX 1 17000 mg surpasses all existing products in terms of potency and effectiveness.
Rigorous testing and medical doctors confirm SDX 1 as a formidable treatment option, infused with natural SPOROLIFE and HYDROGEN ANTIOXIDANT technology. The SDX 1, also the largest in its category, has been a game-changer in providing relief and vitality.
Its unique formula offers exceptional analgesic and anti-inflammatory properties, effectively alleviates stress, and provides a soothing calm along with many other health benefits to users.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Price Adjustment: Price increase in 2024.
October 12, 2023, Kenrico today announced price changes across all their products.
Due to the commodity price increase and the significant drop of Japanese Yen, Kenrico will be making a price adjustment to all Kenrico products, effective Monday, January 1, 2024.
The price adjustment is expected to remain between 10% to 20% of the current prices. The 10% to 20% of price increase is still below the impact of the 200% increase to the commodity prices and the 50% drop of the Japanese Yen against most major currencies.
Until December 31 2023, Kenrico Distributors can still purchase Kenrico products at their current prices and obtain near 50% discount after currency conversion due to the significant drop of Japanese Yen.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Bigger And Better: The New Mega Gold Edition SDX 1 16000 mg
July 4, 2023, Kenrico today revealed the new Mega Gold Edition SDX 1 16000 mg, the biggest patch in the universe, to the medical community.
The new Mega Gold Edition SDX 1 16000 mg is developed by the medical team at Kenrico to free people from world's health problem. The number of diseases and illnesses has continued to grow in number and strength.
Medical statistics show that there is only 1 cure for every 20 diseases. The elderly is the most susceptible to develop chronic illnesses because the cures are not made to suit the physical changes of their age.
The immense size of the new Mega Gold Edition SDX 1 16000 mg is developed to amplify the healing result by expanding the nerves contact points. Additionally, its immense capacity is developed to make the curing effects long-lasting.
"According to the medical data, people's feet get wider and may increase in size with age. The tissues that hold the nerves become less tight causing the increased width and sagging of the arches.
These expanded nerves can continually be treated with the administering the SDX 1 16000 mg as the SDX 1's expanded surface area provides continues contact with the expanded tissues, " said Watanabe M.D., Ph.D, deputy director and Kenrico senior head researcher.
"Furthermore, the SDX 1 with its large capacity of 16000 mg acts like an afterburner where it continues to provide healing effects beyond the normal application time. This benefits people who require extra attention to their health."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Mandatory Requirement: Sporolife
January 24, 2023, Kenrico today announced that Sporolife has been approved as mandatory use in skin patches for regulating blood and lymphatic circulation system.
The approval is based on the data from the CT Scan Diagnosis clinical trial, also known as CTMED575. The major efficacy outcome measure was body weight percentage, blood and lymphatic circulation through CT scan with patients' feedbacks.
In patients who was administered patches that contained Sporolife, Sporolife promoted better sleep quality, reduced body fat, improved blood and lymphatic circulation that led to the cure of many health symptoms, versus non-Sporolife patches.
Non-Sporolife patches were found to be ineffective and the cause of the increase of body fat, blockage of the lymphatic system (lymphedema), stress, and numerous health symptoms.
"The clinial trial, CTMED575, confirmed the mandatory requirement of Sporolife in skin patches by the European Authority. The result proves that not only Kenrico products are effective, they are 100% safe for use in treatment," said Watanabe M.D., Ph.D, deputy
director and Kenrico senior head researcher. "Sporolife is produced exclusively by Kenrico in Japan for all Kenrico Distributors in the world."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2022
New Large-scale Clinical Study In Progress: CTMED575
October 1, 2022, following the clinical success of 3D Neuroimaging & Blood Analysis: TR-MED964 that confirmed the positive effects of Sporolife,
a new large-scale, double-blind clinical study has been sponsored by Kenrico to further investigate and to verify the importance of Sporolife in regulating the lymphatic system.
Utilizing the latest CT (Computed Tomography) scanning, random subjects are treated with patches with Sporolife and Diamonite vs without.
CTMED575 has been scheduled to run until December 2022. The result of the clinical study that contains series of detailed CT scan images will be published in January 2023.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
The Golden Age
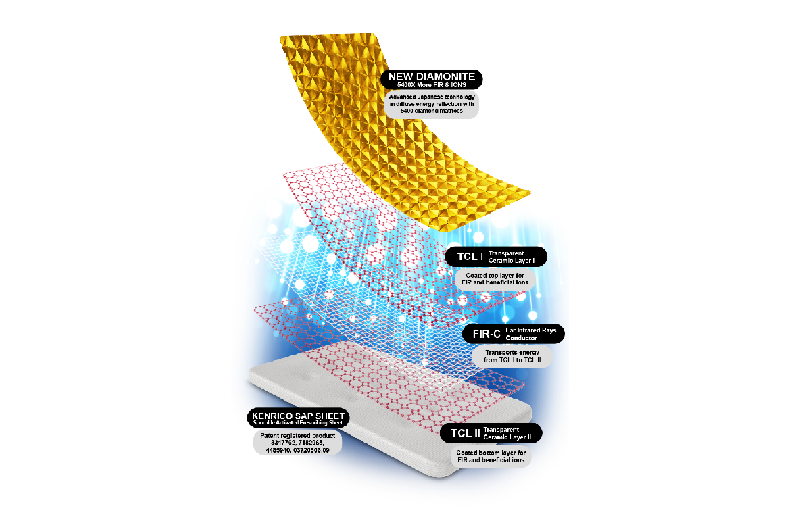
September 17, 2022, the medical world is again put in awe as Kenrico today announced two revolutionary and life changing products in the form of Supreme Gold Edition TRMX 5 50th Anniversary 8500 mg and Master Edition SPOROCYN 4000 mg.
Since being awarded CE Certification with the newest Medical Device Regulation (MDR) in 2021, Kenrico has boosted their research and development to advance their medical technology and to continue changing people lives for the better.
The Supreme Gold Edition TRMX 50th Anniversary marked the 50th years of combined effort between Kenrico, healthcare professionals, and loyal Distributors across the world in improving lives. The product is developed with DIAMONITE™, an individually
constructed five thousand four hundred diamond domes for a superior healing through diffuse energy reflection. At the same time, Kenrico also has developed the Master Edition SPOROCYN 4000 mg - a product that can put a stop to all illnesses and diseases transmitted through
tick or other insect bites.
"These two new life changing products are our humble way of saying thank you to our team of dedicated health professionals and loyal distributors in changing the world." said Nurman S, the President of Kenrico.
"By combining medical knowledge gained through higher learning, dedication gained through fairness and never-give-up attitude, and continuous strive for excellence, we welcome another Golden period of success."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2021
Setting A Gold Standard
November 9, 2021, Kenrico today announced that they have been awarded the latest CE Certification with the newest Medical Device Regulation (MDR). CE Certification under the newest regulation of MDR 2017/745 is a legal requirement that is mandatory in Europe to ensure
that the products are manufactured responsibly, are effective for treatment, and are continuously monitored to ensure the safety of the consumers.
The CE Certification was awarded for several reasons. Kenrico manufacturing facilities are operated responsibly under GMP and ISO 9001.
Their products are manufactured under medical patents, contains patented Sporolife, and have been confirmed safe and effective with clinical studies. Furthermore, their pre and post market surveillance system has been adopted and operated by the Medical Doctors at Kenrico
to ensure the safety of their products.
"Transitioning from the old MDD to the new MDR 2017/745 for the CE Certification was no easy task. But our dedication and commitment in putting patients first has guided us to achieve this victory." said Nurman S, the President of Kenrico.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Chasing Zero
January 5, 2021, Kenrico today announced that upgrades to all Kenrico facilities are in progress until January 31st, 2021. Starting February 1st 2021, all Kenrico facilities and their products are germs free to meet their new policy of zero tolerance towards germs.
Due to the upgrade, their production and shipping are delayed until February 1st, 2021.
"We wanted to further upgrade our quality by having our products germs free starting this year." said Nurman S, the President of Kenrico. "The upgrade will significantly increase our quality and meet our zero tolerance policy towards germs."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2020
Kenrico Announces TRMX LIFE PLUS.
October 1, 2020, Kenrico today announced the launch of TRMX LIFE PLUS with Sporolife and self-stick technology. Kenrico continues to surprise with its pioneering product.
"People suffering from pain the hands and arms can now rejoice." said Suzuki Akio, MD, pharmacologist, Kenrico. "The TRMX LIFE is the first ever patch in the world that attaches directly to the wrist that alleviates pain, inflammation, numbness, tingling, and
any discomfort in the hands and arms. Clinical study with a thousand participants has confirmed the effectiveness of the TRMX LIFE PLUS."
Watanabe MD, Kenrico senior head researcher, said "Sporolife is truly a gift from nature. Not only it pulls toxins from the lymphatic system like a vacuum cleaner, it cures pain, inflammation, and stress as well."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Kenrico Announces TRMX LIFE.
June 1, 2020, Kenrico today announced the launch of TRMX LIFE with Sporolife and self-stick technology. Kenrico continues to lead as the largest skin patch manufacturer in the world.
Watanabe MD, Kenrico senior head researcher, said "in response to the COVID situation, Sporolife X has been added into the TRMX LIFE. SPOROLIFE, as required by medical regulation, is important in promoting detoxification, deep relaxation, creativity, intuitive insights, productivity, intelligence, and positive thinking.
The addition of Sporolife X provides TRMX LIFE with additional antigerm effects. Furthermore, TRMX LIFE is a self-stick pad that removes the need of using separate adhesives. Users can continue to benefit from using our products without the stress and the discomfort of using separate adhesive."
"The launch of TRMX LIFE and its self-stick technology prove Kenrico's continued leadership and commitment to deliver highly effective treatments for their patients." said Nurman S, the President of Kenrico.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2019
Kenrico Sets New Sales Record.
December 29, 2019, Kenrico today announced the financial results for its fiscal 2019 fourth quarter ended on December 29, 2019. The Company posted a record sales of 148 million SAP Sheets worldwide with 99.9% of market share, an increase of 10 percent from the previous year.
"We concluded a groundbreaking fiscal 2019 with our highest earnings, fueled by the growing demands of Sporolife and accelerating sales of the Supreme Gold TRMX 4 Original, Diamond Hybrid Edition DHE 30th Anniversary, and Hot Pink Edition WRX-2i 30th Anniversary,"
said Nurman S, Kenrico's President. "A proven Japanese-made medical product is in high demand and with our Japanese quality, we're confident that we can meet the growing demands and exceed the expectations of our customers in the coming years."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
European Authority has mandated the use of SPOROLIFE.
May 31, 2019, Kenrico announced today that the European Authority, a FDA-level European body,
requires SPOROLIFE to be included in the composition of a skin patch for detoxification purpose. Sporolife is essential for detoxification, deep relaxation, creativity, intuitive insights, productivity, intelligence, and positive thinking.
A skin patch without Sporolife, as proven by clinical study, can cause toxins to backflow into the lymphatic system and cause clogging of the lymphatic tubes. A skin patch claimed to detoxify without SPOROLIFE is not permitted to be sold to consumers.
"This mandate confirmed that the role of SPOROLIFE is important in promoting detoxification, deep relaxation, creativity, intuitive insights, productivity, intelligence, and positive thinking," said Suzuki Akio, MD, pharmacologist, Kenrico. "SPOROLIFE is the secret of why Kenrico SAP Sheet is loved by millions of people because it is very effective in curing illnesses, diseases, and disorders."
Watanabe MD, Kenrico senior head researcher, said "The wrong perception was that all products claimed to detox would would work as it claimed. In fact, skin patch claimed to detoxify without SPOROLIFE has been shown to provide negative health effects and to cause poisoning in numerous clinical studies. We applaud the decision of the Authority in requiring the use of SPOROLIFE."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
EPO granted a patent for Kenrico SAP Sheet.
May 16, 2019, Kenrico announced today that the European Patent and Trademark Office has granted a patent for Kenrico SAP Sheet. Kenrico SAP Sheet is now a patent registered product for distribution to all European countries.
"The patent shows that our product is truly unique with exclusive rights granted by a government," said Nurman S, the President of Kenrico. "We translate our ingenuity into intellectual assets to protect our continuous innovation and our commitment to our customers."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2018
The Supreme Gold Edition TRMX 4 Original ® is ten (10) times more effective.
August 1, 2018,
Kenrico today announced positive result from the latest large-scale clinical study.
Result from the clinical study showed a significant result of TRMX 4 Original in heavy metals cleansing, cognitive, social skills, creativity, and intelligence enhancement. TRMX 4 Original provides significant improvement from the TRMX-3 30th Anniversary: 10.26 times the improvement of total alpha brainwaves, 1.55 times the improvement of maximum alpha brainwaves, and 3.09 times the improvement of SE index (TR-MED964).
Nurman S, the President of Kenrico, said "This is the first time a new treatment can be enhanced up to 10 times the effectiveness of the previous treatment. It shows Kenrico's continued leadership and commitment to deliver highly effective treatments."
Watanabe MD, Kenrico senior head researcher, said "At the core of our research program is a commitment to investigate the potential of Supreme Gold TRMX 4 Original. These important data propelled us to continue to research and to provide the best treatment for all patients suffering from heavy metals poisoning, severe depression, cognitive impairment, learning disabilities, or for people who just want to improve their creativity, intelligence, and social skills."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2017
The Supreme Gold Edition TRMX 4 Original ®
The Diamond Hybrid Edition DHE 30th Anniversary ®
April 31, 2017,
Kenrico today announced that the Supreme Gold Edition TRMX 4 Original and the Diamond Hybrid Edition DHE 30th Anniversary have been approved for market release. The inclusion of Hydrogen Antioxidant in TRMX 4 Original and DHE 30th Anniversary is found to triple the effectiveness
of the original treatment.
"The TRMX 4 Original and the DHE 30th Anniversary are the remarkable progress that are being made against illnesses, diseases and disorders." said Watanabe, MD, Kenrico senior head researcher. "We highlight our clinical breakthrough with our continuous commitment to our customers and patients."
The Supreme Gold Edition TRMX 4 Original provides 300% more analgesic, anti-inflammation and anti-stress properties (world-wide patents 7182965, 4485940, 03720506.09) and has been proven highly effective to enhance cognitive, social skills, creativity and intelligence.
The Diamond Hybrid Edition DHE 30th Anniversary breaks down unwanted fat pockets, removes water retention, shrink the fibrous bands that contribute to dimpling of the skin, and tightens the overlying skin by stimulating collagen formation with no diet, no pills, no exercising and no plastic surgery.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2016
Hydrogen Antioxidant
October 27, 2016, Kenrico today announced new finding conducted by Kenrico scientist on the discovery and development of Hydrogen
Antioxidant, a negatively charged hydrogen with natural antioxidant properties that improves lipid and glucose metabolism, reduces inflammation, allergies, and rapid aging. Hydrogen Antioxidant expands and contracts the wall of Sprorolife that causes it to absorb more heavy metals, toxins and pesticides.
"The development of new activated ingredient for treatment of inflammation and rapid aging has long been a focus of biomedical research." said Suzuki Akio, MD, pharmacologist, Kenrico. "Hydrogen Antioxidant supercharges Sporolife, and more patients suffering from heavy poisoning can benefit from the treatment."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2015
Large-Scale Clinical Trials
February 14th, 2015, Kenrico today announced the successful clinical trial of the Supreme Gold Edition TRMX-3 30th
Anniversary.
The clinical trial of TRMX-3 30th Anniversary represents the largest and most intensively studied Kenrico Sap Sheet over the past decade, with more than 200 patients randomized. The clinical trial confirms the effectiveness of TRMX-3 30th Anniversary in increasing relaxation while enhancing productivity, intelligence, and positive thinking.
"The result proves why patients continue to select our Kenrico Sap Sheet to help treat their health problems," said Suzuki Akio, MD, pharmacologist, Kenrico. "I cannot stress enough, how important it is to use TRMX-3 30th Anniversary to enhance your health. Not only it removes heavy metals, excess uric acid and oxalic acid that are responsibles for kidney problems, TRMX-3 30th Anniversary promotes relaxation, improves productivity, intelligence and positive thinking too. These factors are essential for all treatment to be successful."
Full report of the clinical trial can be reviewed at: https://www.kenrico.com/media/tr-med742/
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2014
30 Years of Changing Lives
June 5th, 2014, Kenrico today announced that the anniversary edition of the Supreme Gold Edition (TRMX-3) will be developed at the Kenrico
Manufacturing Center in Shimada, an advanced production facility certified with GMP, ISO 9001:2008 and ISO 1400
The site is the second Kenrico's factory in Japan which is also the center for the research and development of new pharmaceutical technologies.
"We have a very clear understanding of medical practitioners' expectations around the world to have an effective and proven treatment for curing illnesses, diseases and disorders. Each day millions of families count on TRMX-3 to help them be able to live a healthy life," said Suzuki Akio, MD, researcher, Research & Development, Kenrico. "We dedicate the anniversary edition of TRMX-3 to all health practitioners in the world who have trusted us to continue to save and improve lives for more than 30 years."
TRMX-3 ® is a medical treatment based on Japanese toxins cleansing technology (International patent 8317762, 7182965, 6793945, 6413556) invented by Suzuki, M.D (Pharmacologist, Kenrico) and Nurman S (Biophysical Scientist, Kenrico) designed to help treat the underlying cause of internal and external inflammation and immune response of illnesses from toxins.
The 30th Anniversary Edition of TRMX-3 contains almost twice the amount of Sporolife, the only proven organic compound in the world that binds various substances such as heavy metals, toxins and pesticides like a sponge. Its antimicrobial activity is also suitable as pharmaceutical composition for treating a microbial disease and heavy metals poisoning and as a cosmetic composition for treating age-related or sunlight-induced wrinkle formation.
Kenrico Sap Sheet has been evaluated in many major clinical outcome studies: Brain Wave Research (2003), Far Infrared Research (2004), Negative Ion Research (2004), Heavy Metals Research (2005), LDL-Cholesterol and Triglycerides Research (2006), Lifestyle and Blood Analysis (2006-2007), Miscroscopic Research (2008), Effects of ZEO Pads on Heavy Metals Detoxification (2009), DEFENSOR I (2011) and DEFENSOR II (2013).
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Certificate from Japan National Tax Agency (NTA)
April 23rd, 2014, Kenrico today announced the Japan National Tax Agency (NTA)'s award of tax compliance
excellence. Tax compliance excellence certificate is awarded exclusively to Japanese corporation in good standing with corporate's tax history of 10 years or more.
"Our reputation and the trust that our customers have in us depend on how we run our company responsibly," said Nurman S, biophysical scientist, Director, Kenrico. "Hence, proper accounting, tax filing, infrastructures, and all important aspects of running a trustable business are important to assure successful business with our clients for years to come. I am proud to say that Kenrico has been awarded with tax compliance excellence."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Ion Shower Head LE (Limited Edition)
January 12th, 2014, Kenrico today announced the forthcoming Ion Shower Head LE (Limited Edition), which will be the
flagship model of its next generation of shower heads. It will arrive as the successor to the Ion Shower Head EX, with improvements on the previous model's ergonomic feature, material, durability and effectiveness.
As Kenrico's new flagship model, the Ion Shower Head LE (Limited Edition) features a new crystal-clear transparent look, new aqua aura crystals, more hot spring minerals, better ergonomic grips, and a more advanced water jet system (micromist). The forthcoming Ion Shower Head LE shower head will retain many of the well regarded feature of previous model, including high quality natural crystals, great beneficial ions and far infrared rays.
"Ion Shower Head LE will further expand the possibilities for users who demand the best possible results from their everyday shower," said Watanabe, MD, Kenrico senior head researcher. "The Ion Shower Head LE represents a concentration of Kenrico's advanced shower head technologies and legacy as leader in stress and diseases-related treatment. The LE will build upon the success of the acclaimed Ion Shower Head EX, a shower head highly regarded for its transparent look, high quality crystals, and hot spring minerals with beneficial ions and infrared rays."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2013
Diamond Hybrid Edition (DHE ®) Confirmed Effective To Burn Fats & Cellulite
September 9th, 2013, Kenrico today announced the completion of an open-trial clinical
study. The clinical study over the period of 30 days confirmed the effectiveness of Diamond Hybrid Edition (DHE ®) in eliminating excess fats and cellulite.
DHE ® is a medical treatment based on Japanese toxins cleansing technology (patent 8317762, 7182965, 6793945, 6413556) invented by Suzuki, M.D (Pharmacologist, Kenrico) and Nurman S (Biophysical Scientist, Kenrico) designed to help cleanse the body from toxins and to help the body disperse and eliminate fats and cellulite while at a rest.
"DHE is the key to remove excess fats and cellulite without requiring much effort on your part," said Watanabe, MD, Kenrico senior head researcher. "From now on you can forget wearying diets, exhausting exercises, taking dangerous diet pills and being in fear of weight rebound. DHE makes you fit and lets you get your rest at the same time."
Kenrico Sap Sheet has been evaluated in many major clinical outcome studies: Brain Wave Research (2003), Far Infrared Research (2004), Negative Ion Research (2004), Heavy Metals Research (2005), LDL-Cholesterol and Triglycerides Research (2006), Lifestyle and Blood Analysis (2006-2007), Miscroscopic Research (2008), Effects of ZEO Pads on Heavy Metals Detoxification (2009), DEFENSOR (2011), and DEFENSOR II (2012).
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
A Successful Phase 4 Clinical Trial of TRMX-3 ®
March 31st, 2013, Kenrico today announced the successful completion of the phase 4 clinical trial.
Researchers presented results from the DEFENSOR II after clinical trial sessions over the span of ninety-six weeks. All long-term and short-term treatment utilizing The Supreme Gold Edition (TRMX-3 ®) provides a very significantly boost to the
physical and mental health.
TRMX-3 ® is a medical treatment based on Japanese toxins cleansing technology (patent 8317762, 7182965, 6793945, 6413556) invented by Suzuki, M.D (Pharmacologist, Kenrico) and Nurman S (Biophysical Scientist, Kenrico) designed to help treat the underlying cause of internal and external inflammation and immune response of illnesses from toxins.
"We are pleased to present new results of the TRMX-3 ® to the scientific community that we believe will be valuable in searching for the ultimate key to cure many diseases," said Suzuki Akio, MD, researcher, Research & Development, Kenrico. "DEFENSOR II enables our researchers and scientific partners to understand how significant and how effective TRMX-3 ® is, in curing many diseases."
Kenrico Sap Sheet has been evaluated in many major clinical outcome studies: Brain Wave Research (2003), Far Infrared Research (2004), Negative Ion Research (2004), Heavy Metals Research (2005), LDL-Cholesterol and Triglycerides Research (2006), Lifestyle and Blood Analysis (2006-2007), Miscroscopic Research (2008), Effects of ZEO Pads on Heavy Metals Detoxification (2009), and DEFENSOR (2011).
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2012
New Distributorship Opportunity
November 30th, 2012, Kenrico announced today the availability of strategic partnership through patents, trademarks or brand
names licensing. The strategic partnership enables Kenrico to continue to expand and provide the best and the safest medical products for their clients.
"In the last month alone, we received hundreds of inquiries from companies that were interested in partnership with us which unfortunately had to be turned away due to the unavailability of licensing at that time," said Nurman S, biophysical scientist, Director, Kenrico. "The recent patent approvals of toxins cleansing technology (patent 8317762) have increased the value of our products 1000%. After much consideration, we felt it is our obligation to extend the value to partners that include our present distributors through licensing. Together with our partners, our products will reach part of the worlds that were not available to us previously. We will be able to provide more cure to additional millions of people."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Kenrico Granted Patent Approval For Detox Pads
November 23rd, 2012, US Patent Office announced today the successful application for the grant of a key US and
International Patent for Kenrico's toxins cleansing technology, popularly known as Sap Sheet, Detox Pads or Detox Patches. This signals the achievement of patent coverage for Kenrico Sap Sheet in the world.
Kenrico Sap Sheet is active against a wide range of illnesses ranging from heavy metals poisoning, arthritis, hypertension, fibromyalgia, diabetes and symptoms such as fatigue, headache, stress, slow learning, and other range of symptoms. The new patent provides protection around Sap Sheet as wastes and toxins cleansing, detoxification, blood circulation, physical and mental improvements.
"We are pleased that our top, highly effective product for the treatment of many illnesses, Kenrico Sap Sheet, has achieved world wide recognition in terms of its value and effectiveness" said Nurman S, biophysical scientist, Director, Kenrico. "Our partners with hospitals and medical clinics, including our authorized distributors all over the world, will be pleased to learn the excellent progression of our product."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
DEFENSOR II Study of TRMX-3 In Progress
March 1st, 2012, Kenrico today announces the continuation of the DEFENSOR Study (DEFENSOR II). The
top results of DEFENSOR Study in January confirmed the effectiveness of The Supreme Gold Edition (TRMX-3) to provide cure against pain, inflammation, physical and mental stress. DEFENSOR II (Determining the EFficacy of the MENtal and PhySical
BOosting with SpoRolife), a phase 4 clinical trial, has started its preparation since May 2011 and will be analyzed in a very large scale of participants.
TRMX-3 showed that its analgesic and antidepressant properties significantly improved body health in the fight against pain, inflammation and stress, compared to standard of care. Improvement from fatigue, ability to wake up feeling rested, increased energy and stamina, improvement from joint pains, leg aches, body aches, quality of facial skin, overall feeling of well being, quality of mental focus and concentration and increased confidence were reported by the participants.
"TRMX-3 has set a very high bar in the efficacy of all physical and mental stress treatments" said Suzuki Akio, MD, researcher, Research & Development, Kenrico. "The continuation of the study, DEFENSOR II, enables our researchers and scientific partners to help better understand the profile of this treatment in specific patients populations and to determine the next steps, including improved prescribing and regulatory filings."
Kenrico Sap Sheet has been evaluated in many major clinical outcome studies: Brain Wave Research (2003), Far Infrared Research (2004), Negative Ion Research (2004), Heavy Metals Research (2005), LDL-Cholesterol and Triglycerides Research (2006), Lifestyle and Blood Analysis (2006-2007), Miscroscopic Research (2008), Effects of ZEO Pads on Heavy Metals Detoxification (2009) and DEFENSOR (2011).
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Data from DEFENSOR Study of TRMX-3 ® Presented
January 11th, 2012, Researchers at Kenrico today presented and published official results
from
DEFENSOR (Determining the EFficacy of the MENtal and PhySical BOosting with SpoRolife). The results from DEFENSOR were presented after successful clinical trials.
The official report of DEFENSOR is now available to all Kenrico's authorized distributors.
In the trial, TRMX-3 ® showed significant improvement of mental and physical health in the performance studied. During the 2-month treatment phase, the study occurred in randomly selected group of first team football players and coaches of Woking Football Club. At the end of the study, statistically significant differences in the performance were recorded. When comparing the results of the football matches during the human clinical trial with the TRMX-3 ® to previous performance without the TRMX-3 ®, a statistical analysis presented a maximum increase of 300% of winning percentages. Improvement from fatigue, ability to wake up feeling rested, increased energy and stamina, improvement from joint pains, leg aches, body aches, quality of facial skin, overall feeling of well being, quality of mental focus and concentration and increased confidence were reported by the participants.
"The results of DEFENSOR provide us some insight into the potential role of TRMX-3 in the fight against diseases, and are certainly worthy of further examination," said Watanabe, MD, Kenrico senior head researcher. "Previous results of clinical trials in heavy metals and toxins extraction should give us greater insight into the effect of TRMX-3 in different group of patients with certain illnesses."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2011
European And World Wide Patent for TRMX-3 ®
July 20th, 2011, Recent global success of Sporolife enables Kenrico to receive European and world wide patent
for Sporolife production.
"TRMX-3 with Sporolife has been a tremendous success. Its impressive performance has exceeded all goals and expectations," said Suzuki Akio, MD, researcher, Research & Development, Kenrico. "Our reputation and the trust that our customers have in us depend on how we conduct ourselves as a manufacturer. Hence, answering to the popular demands of our customers, securing worldwide patent is important to assure continuous Sporolife production for the TRMX-3 for years to come. I am proud to say that Kenrico has been the only manufacturer in the world to produce Sporolife."
The world wide patents cover the use of Sporolife in all applications as an antimicrobial pharmaceutical composition for treating a microbial disease and heavy metals poisoning, a cosmetic composition for treating age-related or sunlight-induced wrinkle formation, a nutritional supplement for vertebrates and a supplemented beverage. Sporolife is able to bind various substances such as heavy metals, toxins and pesticides like a sponge.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Positive Effects of TRMX-3 ® in Latest Clinical Study
May 5, 2011, Researchers today presented results from the clinical study, DEFENSOR
(Determining the EFficacy of the MENtal and PhySical BOosting with SpoRolife) with Kenrico's TRMX-3 ®. In the trial, TRMX-3 ® showed significant improvement of mental and physical health in the performance studied. During the 2-month
treatment phase, the study occurred in randomly selected group of first team football players and coaches of Woking Football Club. At the end of the study, statistically significant differences in the performance were recorded. When comparing the
results of the football matches during the human clinical trial with the TRMX-3 ® to previous performance without the TRMX-3 ®, a statistical analysis presented a maximum increase of 300% of winning percentages. Improvement from fatigue,
ability to wake up feeling rested, increased energy and stamina, improvement from joint pains, leg aches, body aches, quality of facial skin, overall feeling of well being, quality of mental focus and concentration and increased confidence were
reported by the participants.
"These results serve as the foundation for our decision to further develop TRMX-3 ® with Sporolife," said Watanabe, MD, Kenrico senior head researcher. "TRMX-3 ® has been confirmed its effectiveness throughout numerous microscopic and clinical studies. Although, its efficacy in extracting toxins such as heavy metals, reducing pain and stress are widely known, the efficacy of the TRMX-3 ® in boosting mental and physical health that relate to performance has not been confirmed until now. We look forward to continuing to study TRMX-3 ®."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Kenrico's 100% Guarantee
March 20th, 2011, Kenrico announced today that all Kenrico's facilities or factories in Kanaya and Shimada have been declared clear and
not affected by radiation. Kenrico will continue to perform radiation examination for every single batch of manufacturing to eliminate any risk of radiation contamination from Fukushima province. Kenrico is taking all precaution necessary and
guarantee that all products and ingredients manufactured by Kenrico are safe and not exposed to radiation.
"Although Kenrico's factories are geographically located very far and are not affected by the earthquakes, tsunami or radiation in the Fukushima province, Kenrico is taking additional precautionary steps to ensure absolutely no radiation exposure to our products", said Fujita, MD, Quality Assurance, Kenrico. "Every ingredients and every manufacturing batches at Kenrico has been triple inspected and will continue to be triple inspected for every production. We can guarantee and assure our clients that our products are still and always 100% safe and 100% effective. The manufacturing standard of Kenrico is graded as one of the world's best in terms of safety and quality. All of our ingredients are typically grown in-house in Shizuoka province in a closed environment protected by HEPA filters and sensors. During manufacturing, the ingredients are processed in clean-room environment away from dust particles, away from dangerous germs or bacteria and obviously away from static electricity and away from radiation. Kenrico Quality Assurance Teams have been certified by Japanese Ministry of Health and ISO 9001:2000 to perform these inspections."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
New Imprinting Capability for All Products Manufactured By Kenrico
January 11th, 2011, Kenrico announced today that the new imprinting capability is now available
for all products. Imprinting offers flexibility for small to medium companies that seek private labeling on Kenrico's manufactured products. Imprinting also meets the labeling requirement sets by U.S Food and Drug Administration (FDA), European
Authorized Bodies (CE marking) and Health Canada.
"Kenrico has been providing extra capabilities to assist companies develop their own products and marketing strategies," said Nurman S, global director, Kenrico. "Not only we are committed to deliver highly effective products to our consumers, we are fully committed to assist them to reach great success. The imprinting capability is an important milestone in our effort to bring forward an extra option for small to medium companies."
More information of the new imprinting capability is available online at kenrico.com/japan/factory.html.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2010
New Kenrico ® Forever Alkaline Water Stick - A Solution In Fighting Diseases
December 1st, 2010, Kenrico today announced the launch of Kenrico Forever
Alkaline Water Stick Purifier, a practical solution in fighting diseases, aging and magnesium deficiency.
Kenrico Forever Alkaline Water Stick Purifier is a compact cylindrical tube that fits in water bottle, thermos, and other containers on the go. It is the most practical and most effective water purification system. Better still, it lasts forever.
"With the progress of our research in miniature and nanotechnology, this mini cylindrical stick was finally developed with great success. It creates oxygen-rich water, reduces bacteria and supplements magnesium without human effort and guaranteed to work forever under normal use. It can be used repetitively without any limit. Just place it in a water bottle and thermos, fill them with water or beverages, and that's it", said Suzuki, Akio, MD, researcher, Research & Developement, Kenrico. "Although most people understand the importance of drinking alkaline or oxygen-rich water, magnesium deficiency is often forgettable. Magnesium deficiency is widespread, but often under-detected because the role of the magnesium, not only is a plasma, but intercellularly. A significant portion of symptoms related to many chronic disorders are caused by a magnesium deficiency like allergies, fibromyalgia, migraines, etc. The Forever Alkaline Water Stick Purifier adds this missing magnesium into water and more. It has the capability to cure most common diseases."
More information of The Kenrico Forever Alkaline Water Stick Purifier is available online at https://www.kenrico.com/cure/foreveralkaline.html.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Kenrico To Develop LEXIRIN
May 12th, 2010, Kenrico announced today that they have achieved the capability to produce Anti-Apoptotic composition, LEXIRIN (patent numbers
6,793,945 and 6,413,556)..
LEXIRIN is a medical breakthrough for the treatment of gastrointestinal disorders, deleterious dermatological conditions, immunosuppression or immunodeficiency, reperfusion damage resulting from ischemia, cardiovascular disorders, transplantation, wound healing, tissue rejection and Alzheimer's disease, and the treatment of adverse reactions from medications. Researched by Dr. Bathurst and Foehr, LEXIRIN also may significantly decrease conditions and diseases caused by aging and blocked blood circulation.
"Emerging research and Phase I human clinical trials further confirms its effectiveness and safety," said Watanabe, MD, Kenrico senior head researcher. "Extracted from organic soybean, Lexirin is a key ingredient in treating gastrointestinal and cardiovascular disorders, wound healing and many symptoms related with adverse reactions from medications. Since many diseases are related to apoptosis condition, such as ischemia, using Lexirin with its Anti-Apoptotic property proven to help restore these elements is important."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Kenrico Has Received US & Japanese Patent for TRMX-3 with Sporolife
April 15th, 2010, Kenrico announced today that it has received Japanese patent for
Sporolife use in the Supreme Gold Edition (TRMX-3).
The patent covers the use of Sporolife in all applications as an antimicrobial pharmaceutical composition for treating a microbial disease and heavy metals poisoning, a cosmetic composition for treating age-related or sunlight-induced wrinkle formation, a nutritional supplement for vertebrates and a supplemented beverage. Sporolife is able to bind various substances such as heavy metals, toxins and pesticides like a sponge.
"Both patents are the evidence of Kenrico's continued leadership and commitment to deliver highly effective and absolutely safe treatments to the patients," said Suzuki Akio, MD, researcher, Research & Development, Kenrico. "Meeting our patient's demand is consistent with Kenrico's values as a health care company and is critical to our business operations."
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
TRMX-3 with Sporolife
January 15th, 2010, As a result of successful regulatory approvals in Sporolife production, Watanabe, MD, Kenrico senior head
researcher, said the company has completed the manufacturing of Supreme Gold Edition (TRMX-3) with Sporolife.
"Our goal is to meet our patient's demands for highly effective and safe method in the treatment of heavy metals poisoning. Today, we hope to have achieved that goal by becoming the first company in the world that has successfully manufactured Sporolife for application in alternatives and pharmaceutical drugs. Successful health treatment depends on high quality products that work safely and effectively, and the TRMX-3 is the answer to that demands."
Sporolife is a natural composition made from major component of the tough outer (exine) walls of plants. The natural ingredient is a very significant discovery for its reputed analgesic and antidepressants effects to relieve stress and depression. The patent covers the use of Sporolife in all applications as an antimicrobial pharmaceutical composition for treating a microbial disease and heavy metals poisoning, a cosmetic composition for treating age-related or sunlight-induced wrinkle formation, a nutritional supplement for vertebrates and a supplemented beverage. Sporolife is able to bind various substances such as heavy metals, toxins and pesticides like a sponge.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
2009
Kenrico To Research and Produce Sporolife
December 20th, 2009, Kenrico and Dr. Maack today announced that they have entered into a definitive agreement by which
Kenrico will continue the research and apply the invention in pharmaceutical fields for the benefit of patients with heavy metals poisoning, stress, depression, microbial disease, aging skin and cancer. Dr. Maack is a renowned scientist with
specific expertise in chemistry and microbial-derived biologics.
"At Kenrico we continue to expand our pharmaceutical expertise and manufacturing capability to meet our patients' demands for highly effective and absolutely safe pharmaceutical products," said Watanabe, MD, senior head researcher, Research & Development, Kenrico. "By continuing the research, Kenrico through its distributors will be able to help many more patients that need our utmost care."
The definitive agreement is subject to regulatory GMP approval in Japan for the continuation of the research and setting up an operational facility to produce the Sporolife for application in alternative and pharmaceutical drugs such as kenrico sap sheet, detoxifying capsules, anti-germ disposal facial mask and arigato lotion.
World's First and Most Potent SUPREME GOLD TRMX-3
October 21, 2009, TRMX-3, SUPREME GOLD edition, is the world's first and most potent detoxification pads.
TRMX-3 is impregnated with 8.5 grams of patented formula with 6.00 grams of Tourmaline, an upgrade from 4 or 5 grams in TRMX-1, TRMX-2 and TRMX-2i.
Kenrico is pleased that the regulatory agencies of CE (Conformite Europeenne), GMP (Good Manufacturing Practice) and ISO (International Standard Organization) approved the TRMX-3, bearing their marks, to be safe and effective. TRMX-3 is for use in adults and children above 3 years old for the prevention and relieve of fatigue, headache, double vision, blood pressure, arthritis, rheumatism, skin problems, stress, slow learning, hot flashes due to menopause, and mood swings, Crohn's disease, Fibromyalgia, and heavy metal poisoning.
"Kenrico has been committed to pursuing the use of the TRMX-3 in both males and females since the earlier TRMX-1 was developed over a decade ago," said Suzuki Akio, MD, researcher, Research & Development, Kenrico. "We are pleased that the regulatory agencies agree that the data support the compliance of TRMX-3."
Clinical trials presented to the regulatory agencies in 2003, 2005 and 2008, evaluated the efficacy, immunogenicity and safety of Kenrico pads in adult men and women 21 to 65 years of age. Efficacy in males and females were evaluated in a randomized, double-blind, placebo-controlled trial. A total of 2,620 participants were enrolled and received daily dose of Kenrico pads or placebo. Of these, 1,042 were men aged 21 to 65 years and 1,578 were women aged 21 to 65 years. Hair, blood analysis and brainwave pattern analysis revealed an improvement after continuous 12 to 24 weeks and significant improvement after continuous 2 years of treatment with Kenrico pads.
Kenrico To Launch Carbon-Titanium Adhesives with Easytouch
October 6th, 2009, Kenrico will begin launching Carbon-Titanium Adhesives with EasytouchTM in
both domestic and international markets in the third week of October 2009.
EasytouchTM is a patented method of pads attachment with the adhesives tapes for simple and easy application. Carbon-Titanium Adhesives is a titanium and carbon combination for patients with tendonitis, bursitis, arthritis and immobility.
"Kenrico is pleased that we are now able to supply Carbon-Titanium Adhesives with the EasytouchTM in demanding markets and continue our long-standing commitment to help address unmet medical needs in cardiovascular care," said Watanabe, MD, senior head researcher, Research & Development, Kenrico.
Kenrico Disposal Facial Mask
July 20th, 2009, Kenrico Disposal Facial Mask ® is the first facial mask with carbon titanium and antigerms capabilities. Since 1997, Kenrico Facial
Mask has been used over 10 million people to protect against avian and swine flu. In 2008, Kenrico made a commitment to produce at least 10 million of Kenrico Facial Mask over two years to help address the problem of avian and swine influenza infection.
In July 2009, the first Kenrico Disposal Facial Mask ® with antigerms capabilities were developed.
Kenrico is a global research-driven pharmaceutical company dedicated to putting patients first. Established in 1985, Kenrico currently discovers, develops, manufactures and markets prescription and alternative medicines to address unmet medical needs. The Company devotes extensive efforts to increase access to medicines through far-reaching programs that not only donate Kenrico medicines but help deliver them to the people who need them. Kenrico also publishes unbiased health information as a not-for-profit service. For more information, visit www.kenrico.com.
Kenrico Sap Sheet ZEO, The clinical Trials
March 11th, 2009, New clinical trial verifies the efficacy of Kenrico Sap Sheet ZEO in heavy metals
detoxification.
ISO 14001 and ISO 9001:2000 certification
February 1st, 2009, Kenrico has recently been independently audited and awarded ISO 14001 and ISO 9001 by JISC
(Japanese Industrial Standard). These certifications from one of the most respected bodies in the world signify to customers that they can be sure they are buying from a company that is both environmentally responsible and compliant with environmental
law.
ISO 14001 is the standard for environment certification which supports environmental protection and prevention of pollution. ISO 14001 also ensures that the product is designed with the environment in mind.
A commitment to operating Kenrico in conformance with the ISO 14001 and 9001 standards not only helps to ensure the quality of products and services, but more importantly confirms to all that Kenrico has defined and documented processes that ensure consistent customer satisfaction. Kenrico strives to only provide the best products and to earn respects from its clients.
2008
Long-Term Allergy Relief
December 20th, 2008, FX-2 and AGi Sap Sheets offer a new hope for allergy relief.
New TUV Sap Sheet
September 12th, 2008, Soothing Edition (Aqua Cool TUV) with Tourmaline & 5 soothing herbs. Receive a relaxing, soothing sensation while
detoxing at
the same time. Made in Japan with the highest grade ingredients.
UNICEF Japan And Kenrico, a Partnership To Reach Common Goals
July 9th, 2008, Kenrico pledged cash and Sap Sheet donation to UNICEF Japan. Kenrico and its distributors strive to be
part of a global effort to save, protect and improve children's lives.
Microscopic Research Explained The Efficacy of Kenrico Sap Sheet
June 11th, 2008, Microscopic research conducted by Japanese Food Research
Laboratories offered proof of the high effectiveness of Kenrico Sap Sheet in removing toxins from the body such as heavy metals, uric acid and oxalic acid.
100% Organic Ingredients
June 5th, 2008, Kenrico's organic certification for Kenrico Sap Sheet addresses a growing worldwide demand for organic
ingredients.
Kenrico Sap Sheet Has Passed 2008 Mandatory Safety Testing
March 3rd, 2008, Mandatory Safety Testing further confirmed Kenrico's highest
manufacturing
standard.
2007
Lifestyle & Blood Analysis Clinical Testing Investigated the WRX-2 Sap Sheet
May 29th, 2007, Clinical trials confirmed the efficacy of
WRX-2 Sap
Sheet in restoring body balance and assist in the treatment of diabetes, liver and heart disease
The book, Miracle Patches II
May 20th, 2007, The world's second book about Sap Sheet is the final stage of development.
2006
Clinical Trials Confirmed The Efficacy of CC Sap Sheet
May 30th, 2006, A breakthrough in the treatment of cardiovascular disease with the
CC Sap
Sheet
Excellent Cleaning Result
January 4th, 2006, 100% effectiveness against germs. Kenrico Foot Wipes.
New CC Sap Sheet
January 1st, 2006, New CC sap sheet with Green Tea (Catechin - ECGC and L-Theanine). Total of 15 Sap Sheet to choose from.
2005
Efficacy Test on Kenrico Gentlecare Foot Wipes
November 4th, 2005, Confident testing result from the latest testing of Kenrico Gentlecare Foot Wipes.
Kenrico Gentlecare Foot Wipes are now available
September 3rd, 2005, New foot wipes. Perfect combination with Kenrico Sap Sheet.
Further Research And Medical Test Yielded a New Sap Sheet
July 3rd, 2005, New QU-1 detox pad with Milk Thistle. 14 high quality detox pads to choose
from.
The book, Miracle Patches
July 1st, 2005, The world's first book about Kenrico Sap Sheet, "Miracle Patches..." is now available.
Kenrico's Second Factory is Now Operational
March 20th, 2005, Our second production facility expansion is now complete. Doubling Kenrico's production speed and
delivery.
Clinical Trials Confirmed The Capability of Kenrico Sap Sheet
January 8th, 2005, Clinical trials have been completed to verify the effectiveness of
Kenrico Sap
Sheet in heavy metals detoxification
2004
New KX-2 Sap Sheet
November 11th, 2004, New KX-2 model with Tourmaline and Amethyst. 12 high quality Kenrico Sap Sheet to choose from.
Custom Print Has Been Made Available
July 20th, 2004, Custom print is available for minimum of 100,000 sap sheets.
Continuous Research To Achieve Proven & Superior Quality
June 4th, 2004, Kenrico continuous research in details. Another reason why Kenrico Sap Sheet is
superior.
2003
Kenrico Sap Sheet Has Passed 2003 Mandatory Safety Testing
May 7th, 2003, Microbial Limit further validated the testing result of USP Microbial
Limit Testing.
Kenrico Sap Sheet Has Passed USP Microbial Limit Testing
May 7th, 2003, USP Microbial Limit Testing confirmed the cleanness of Kenrico Sap
Sheet.
Kenrico Sap Sheet Has Passed FHSA Skin Irritation Testing
May 7th, 2003, FHSA Skin Irritation Testing performed on Kenrico Sap Sheet indicated
that Kenrico Sap Sheet is non irritant and absolutely safe for all ages.